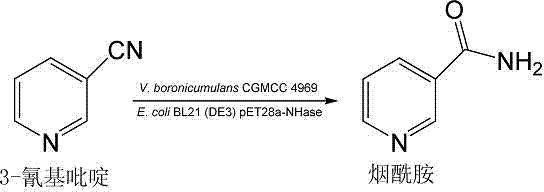
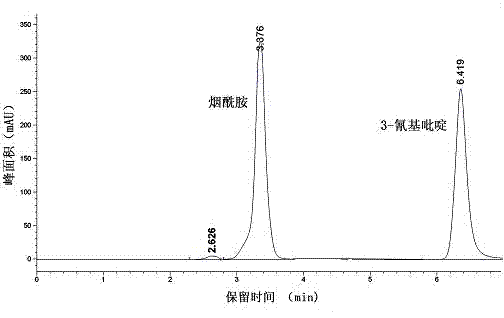
Vaporophage bacterium cgmcc4969 and its application in the biotransformation of 3-cyanopyridine to nicotinamide
A technology of biotransformation and cyanopyridine, applied in the field of microorganisms, can solve problems such as the function of nitrile hydratase that has not been proved by experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Example 1: Isolation, screening, identification and biological characteristics of strains that can biotransform 3-cyanopyridine to nicotinamide
[0017] 1. Strain isolation
[0018] Collect soil from the Xianlin area of Nanjing City, Jiangsu Province, add 1g of soil to 19mL sterile water containing 5 glass beads, shake for 5min, let stand for 10min, take 1ml suspension and add 19ml mineral containing 1% 3-cyanopyridine Salt medium. The composition of the mineral salt medium is: 1.36 g / L KH 2 PO 4 , 2.13g / L Na 2 HPO 4 , 0.5 g / L MgSO 4 ·7H 2 O and 10ml / L metal ion solution, pH 7.5. The composition of the metal ionic liquid: 0.40 g / L CaCl 2 ·2H 2 O, 0.30 g / L H 3 BO 3 , 0.04 g / L CuSO 4 ·5H 2 O, 0.10 g / L KI, 0.20 g / L FeSO 4 ·7H 2 O, 0.40 g / L MnSO 4 ·7H 2 O, 0.20 g / L NaMoO 4 ·2H 2 O and 10.0 mL / L concentrated hydrochloric acid. The samples were cultured in a shaker at 30°C and a rotating speed of 220 rpm for 3 days. Dilute 100μl sample to 10 -3 -10 -5 After that, a plate of...
example 2
[0024] Example 2: V. boronicumulans CGMCC 4969 nitrile hydratase gene cloning
[0025] V. boronicumulans The genomic DNA extraction method of CGMCC 4969 is the same as that of Example 1.
[0026] The four nucleotides involved are: deoxyadenine triphosphate (abbreviated as A), deoxythymidine triphosphate (denoted as T), deoxyguanine triphosphate (abbreviated as G) , Deoxycytosine triphosphate (abbreviated as C). Design and synthesize the upstream and downstream primers of the nitrile hydratase gene. R in the primer indicates that the base at that position is A or G, S in the primer indicates that the base at that position is C or G, Y in the primer indicates that the base at that position is C or T, and K in the primer indicates the base at that position The base is G or T.
[0027] First clone with degenerate primers V. boronicumulans CGMCC 4969 nitrile hydratase alpha subunit gene fragment. The sequence of the synthesized primer NHCo-f is: 5'-GTSGTGGCSARGGCCTGG -3' (SEQ I...
example 3
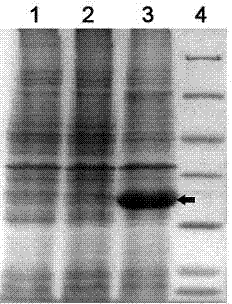
[0032] Example 3: Expression of nitrile hydratase gene and expression of recombinant nitrile hydratase gene cluster E. coli For the biotransformation of 3-cyanopyridine
[0033] 1. Connect the DNA fragment of the nitrile hydratase gene to the plasmid pET28a
[0034] Design primer NHCo-Ef containing EcoRI restriction site and primer NHCo-Er containing XhoI restriction site. The sequence of primer NHCo-Ef consists of 28 nucleotide residues, in order: 5′-GGGGAATTCATGACCGGCCATGACCACT-3 '(SEQ ID No: 13); The sequence of primer NHCo-Er consists of 27 nucleotide residues, in order: 5'-GGGCTCGAGTGCCGCG GGCTCCAGGTA-3' (SEQ ID No: 14). In a sterile 0.2mL PCR thin-walled tube, add 10.8μL sterile water, 2μL amplification buffer, 2μL four deoxynucleotides, 0.5μL primer one, 0.5μL primer two, and 3μL. The genomic DNA solution prepared in step 1, 1μL of DMSO, 0.2μL of Angel DNA polymerase, the total volume is 20μL; put the PCR tube in the PCR machine, and set the conditions as follows: increa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com