Method for preparing mutant escherichia coli capable of simultaneously utilizing glucose and xylose
一种大肠杆菌、葡萄糖的技术,应用在基于微生物的方法、使用微生物的方法、生物化学设备和方法等方向,能够解决缺少除去、消除CCR等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of mutant E. coli by promoter replacement and evolutionary adaptation
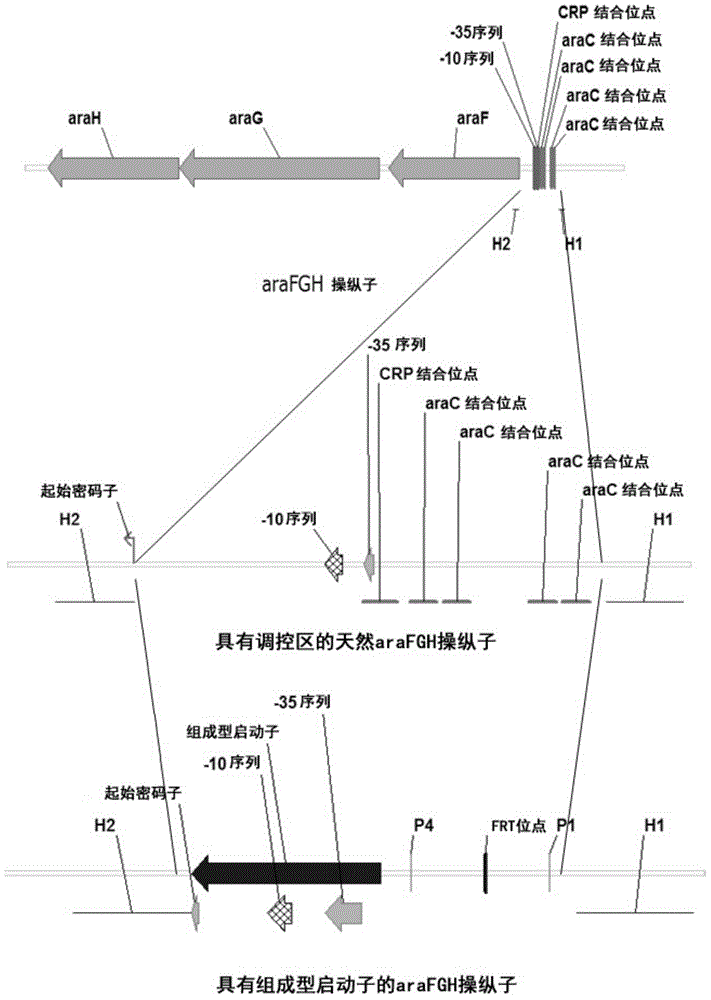
[0051] Mutant E. coli was prepared by replacing the inducible promoters of the araBAD operon, araFGH operon, araE gene, xylAB operon, and xylFGH operon of wild-type E. coli with the respective constitutive promoters in the following manner.
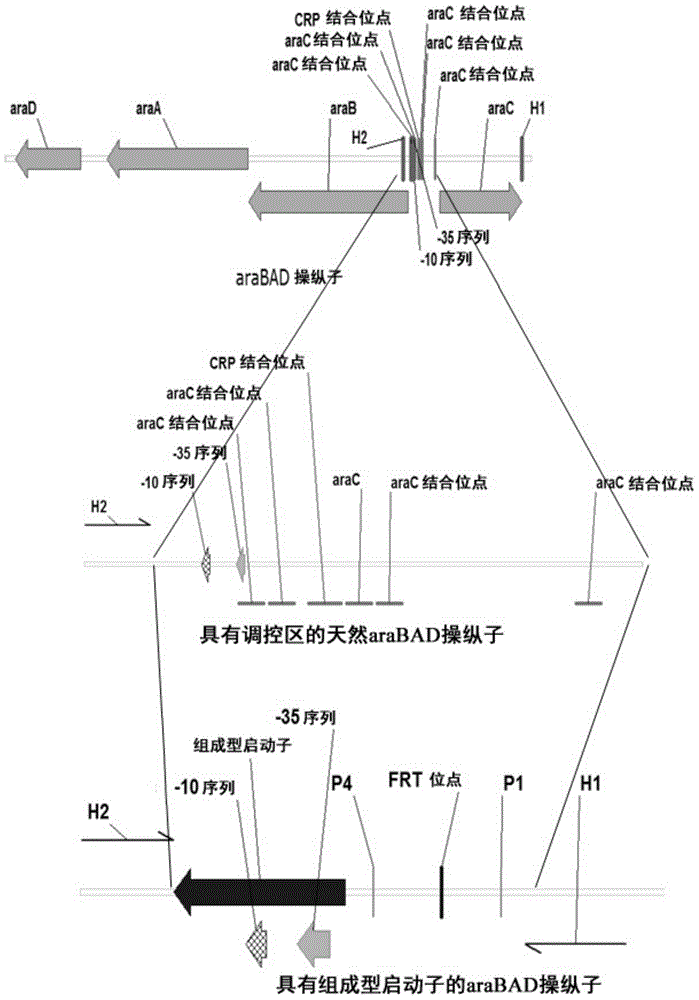
[0052] Inducible activation of araBAD operon, araFGH operon and araE gene substituting the respective constitutive promoters
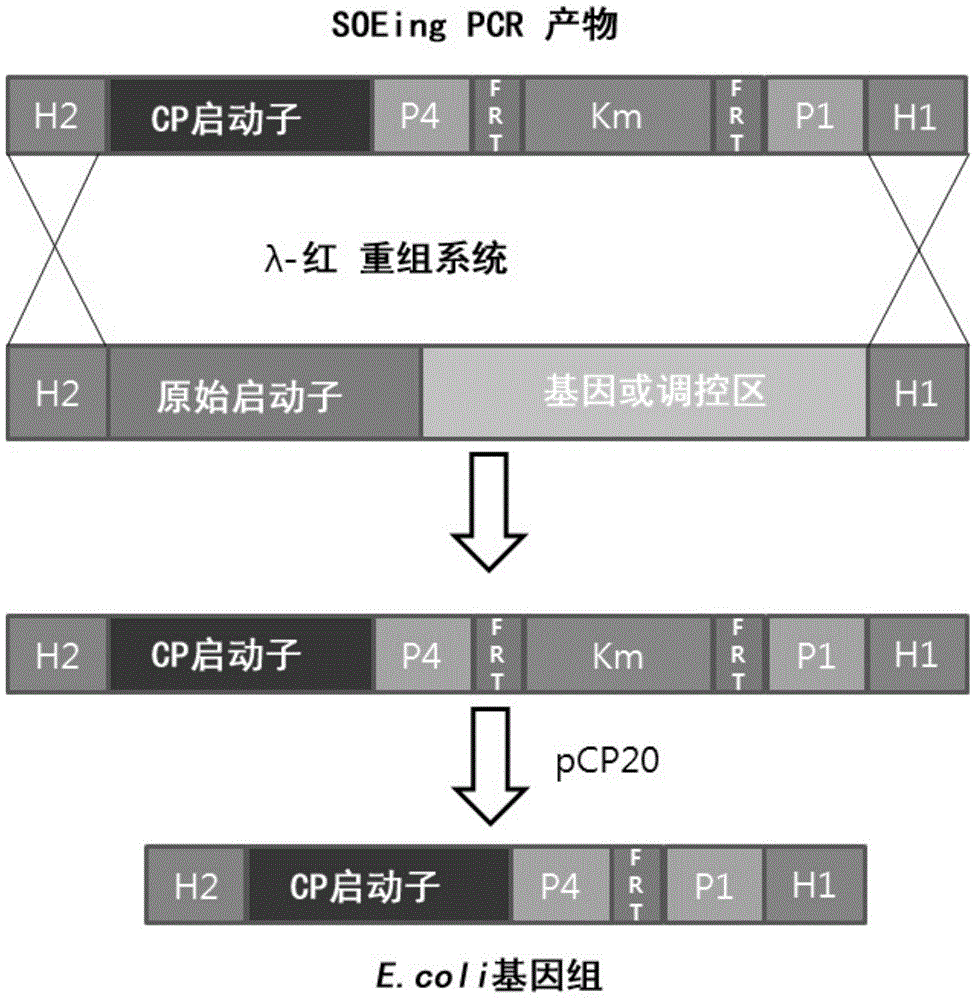
[0053] The λ- Red recombination system, the inducible promoter (SEQ ID NO:1) of the araBAD on the chromosome of E.coli MG1655 is replaced by the constitutive CP25 promoter (SEQ ID NO:6) (see figure 1 ).
[0054] In short, such as literature [Datta, S., N. Costantino, et al. (2006). "A set of recombineering plasmamids for gram-negative bacteria." Gene 379 (0): 109-115; Datsenko KA et al ., Proceedings of the National Academy of Sciences of the United States of America, 97(12), 6640-6645, 2000; and Cherepano...
Embodiment 2
[0079] Example 2: Preparation of mutant E. coli by promoter replacement and evolutionary adaptation in minimal medium of arabinose and xylose
[0080] In addition to using the bacterial strain obtained in the embodiment in the basic medium of arabinose and xylose (containing arabinose 2g / L, xylose 2g / L, disodium hydrogen phosphate 6.78g / L, dihydrogen phosphate Potassium 3.0g / L, sodium chloride 0.5g / L, ammonium chloride 1.0g / L, magnesium sulfate 2mM and calcium chloride 0.1mM M9 basal medium) cultured in 50 days to replace xylose basal medium, according to The same method as in Example 1 was used to prepare mutant E. coli strains.
[0081] Evolved in minimal media with arabinose and xylose after replacing the inducible promoters of the araBAD operon, araFGH operon, and araE and xylAB operon and xylFGH operon with constitutive promoters The adapted strain was named "AXcpAX50".
Embodiment 3
[0082] Embodiment 3: the preparation of the bacterial strain that knocks out ptsG
[0083] Preparation of wild-type E.coli with knockout of ptsG
[0084] To examine the molecular mechanism of the mutant strain (AXcpAX50) for CCR elimination, ptsG gene disruption was performed in the chromosome. As previously reported (Nichols et al., Appl. Microbiol. Biotechnol., 2001, 56:120–125), it is known that EIIBC, which encodes the glucose transporter Glc Strains mutated on ptsG utilize both glucose and xylose. To elucidate that the molecular mechanism of the mutant E. coli strain is due to the new mutant factor, wild-type E. coli MG1655 with ptsG deletion was prepared. By replacing the ptsG gene coding sequence with the kanamycin resistance gene in the same manner as described for the promoter replacement in Example 1, and then finally deleting the kanamycin resistance in the same manner as in Example sex genes for ptsG disruption. Thus, E. coli MG1655 in which ptsG was knocke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com