Preparation method and drug carrying method of escherichia coli outer membrane vesicle, and application of outer membrane vesicle in anti-tumor
A technology of Escherichia coli and outer membrane vesicles, applied in the biological field, can solve the problems of hindering the application of OMV, low diffusion efficiency, inconvenient operation, etc., and achieve the effects of inhibiting tumor cell growth, simple operation and reducing invasive ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of E. coli Outer Membrane Vesicles
[0041] (1) Recovery and in vitro culture of Escherichia coli
[0042] Thaw the frozen storage solution of BL21 strain, use the inoculation loop to pick up the bacterial solution, inoculate it on a solid medium containing 50 μg / ml kanamycin, cultivate it in a 37°C incubator for 1 hour, and inoculate a single colony on the solid medium to LB liquid medium containing 50 μg / ml kanamycin, cultured with shaking at 37°C for 1 hour.
[0043] Then the bacterial solution was inoculated into LB liquid medium (which had been sterilized at high temperature) containing 50 μg / ml kanamycin at a ratio of 1:100 by volume, and continued shaking culture at 37°C. When the bacterial OD value (600nm) reached When 0.1, add isopropylthiogalactoside (IPTG) in LB liquid medium, make the final concentration of isopropylthiogalactoside in LB liquid medium be 1 μ g / ml, promote Escherichia coli to secrete extracorporeal Membrane vesicles were culture...
Embodiment 2
[0050] Preparation of E. coli Outer Membrane Vesicles
[0051] (1) Recovery and in vitro culture of Escherichia coli
[0052] Thaw the frozen stock solution of BL21 strain, use an inoculation loop to pick up the bacterial solution, inoculate it on a solid medium containing 50 μg / ml kanamycin, cultivate it in a 37°C incubator for 48 hours, and inoculate a single colony on the solid medium to LB liquid medium containing 50 μg / ml kanamycin, cultured with shaking at 37°C for 24 hours.
[0053] Then the bacterial solution was inoculated into LB liquid medium (which had been sterilized at high temperature) containing 50 μg / ml kanamycin at a ratio of 1:100 by volume, and continued shaking culture at 37°C. When the bacterial OD value (600nm) reached At 0.5, add isopropylthiogalactoside (IPTG) in the LB liquid medium, so that the final concentration of isopropylthiogalactoside in the LB liquid medium is 500 μg / ml, to promote Escherichia coli to secrete extracellular Membrane vesicles...
Embodiment 3
[0061] Validation of E. coli outer membrane vesicles
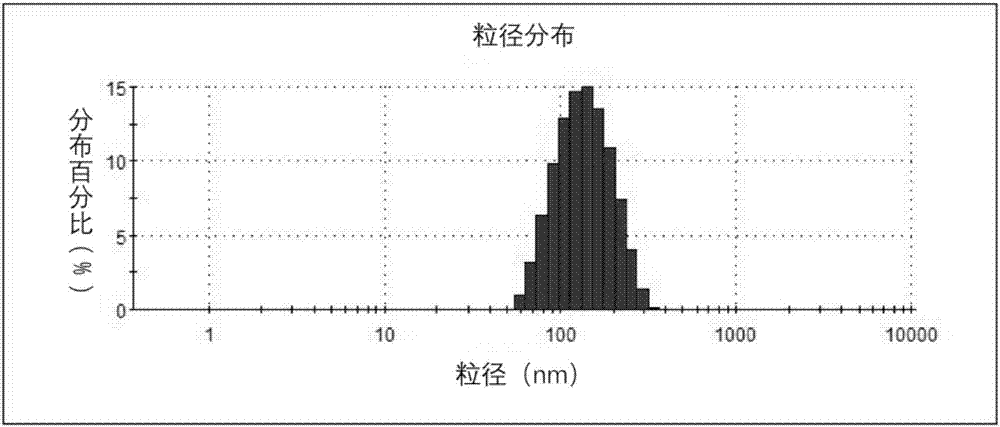
[0062] (1) Particle size measurement of E. coli outer membrane vesicles
[0063] First, use PBS buffer to adjust the outer membrane vesicles synthesized in Example 1 to 100 μg / ml, and measure the size of the outer membrane vesicles under the Malvern laser particle size analyzer. The results are as follows figure 1 shown.
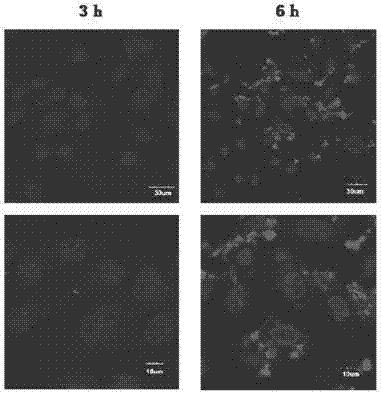
[0064] (2) Morphological observation of outer membrane vesicles
[0065] Take the outer membrane vesicle solution of Example 1, drop it on a special copper grid, and directly observe the size and shape of the outer membrane vesicle under a scanning electron microscope, the results are as follows: figure 2 , image 3 shown.
[0066] (3) Investigate the yield of Escherichia coli outer membrane vesicles
[0067] Take the outer membrane vesicle solution of Example 1, add lysozyme (1 mg / ml), and shake at room temperature for 30 minutes. Subsequently, the BCA protein kit was used to detect the protein c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com