Human heparin binding protein assay kit with high sensitivity and wide detection range
A heparin-binding protein and kit technology, applied in the field of immunodetection, can solve the problems of inaccurate quantification, long time consumption, insufficient sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
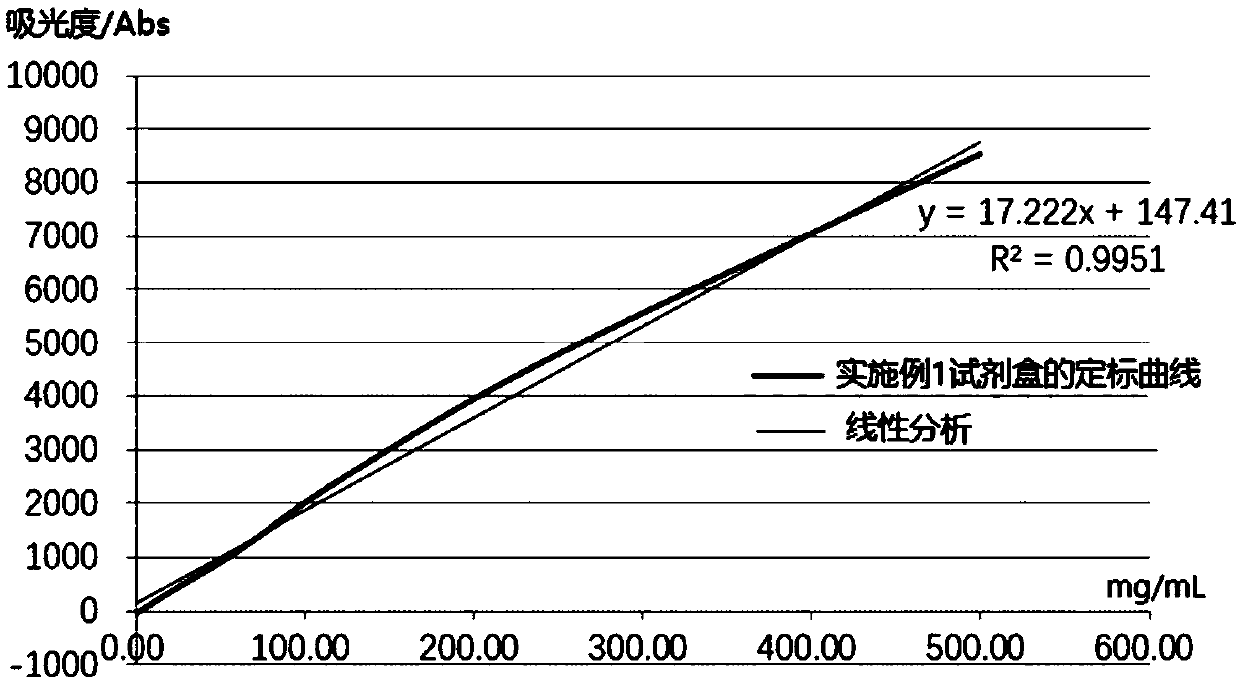
Embodiment 1
[0064] A human heparin-binding protein assay kit, comprising reagent 1, reagent 2 and human HBP calibrator with different concentration gradients; wherein,
[0065] Reagent 1 comprises the following components of final concentration: 20mM HEPES buffer solution (pH7.2), the BSA of 1.5% (w / v), the PEG6000 of 2% (w / v), the polyol of 5% (w / v) Polylysine, DTT of 10mM, KCl of 150mM, Tween-20 of 0.05% (w / v), Krovin300 of 0.1% (w / v), EDTA of 5mM;
[0066] Reagent 2 includes the components of the following final concentrations: carboxyl latex microspheres (average particle diameter is 273nm, microsphere final concentration is 0.015% (w / v)), 0.008% (w / v ) labeled human HBP monoclonal antibody, carboxyl latex microspheres labeled only with human HBP polyclonal antibody (the average particle diameter is 123nm, the final concentration of microspheres is 0.2% (w / v)), 0.045% (w / v) Labeled human HBP polyclonal antibody, 50mM HEPES buffer (pH7.4), 2% (w / v) BSA, 4% (w / v) sucrose, 1% (w / v) treh...
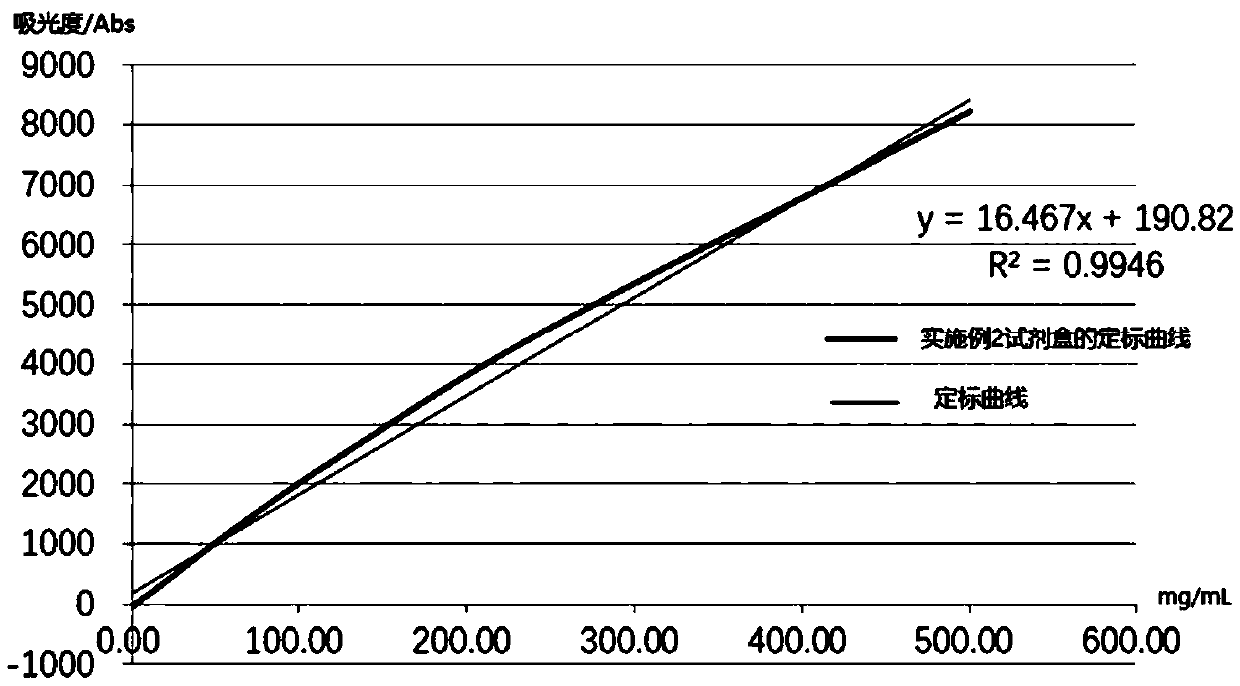
Embodiment 2
[0073] A human heparin-binding protein assay kit, comprising reagent 1, reagent 2 and human HBP calibrator with different concentration gradients; wherein,
[0074] Reagent 1 included the following final concentrations of components: 50 mM MOPS buffer (pH 7.4), 0.5% (w / v) BSA, 2.5% (w / v) PEG8000, 1.5% (w / v) Mw Dextran of 40000, TECP of 5mM, NaCl of 300mM, Tween-20 of 0.05% (w / v), ProClin300 of 0.1% (w / v), EDTA of 5mM;
[0075] Reagent 2 includes the components of the following final concentrations: carboxyl latex microspheres (average particle diameter is 223nm, microsphere final concentration is 0.02% (w / v)), 0.006% (w / v ) labeled human HBP monoclonal antibody, carboxyl latex microspheres labeled only with human HBP polyclonal antibody (average particle size is 88nm, final concentration of microspheres is 0.25% (w / v)), 0.04% (w / v) Labeled human HBP polyclonal antibody, 20mM PBS buffer (pH7.4), 0.5% (w / v) gelatin, 2% (w / v) sucrose, 3% (w / v) trehalose, 50mM chloride Potassium...
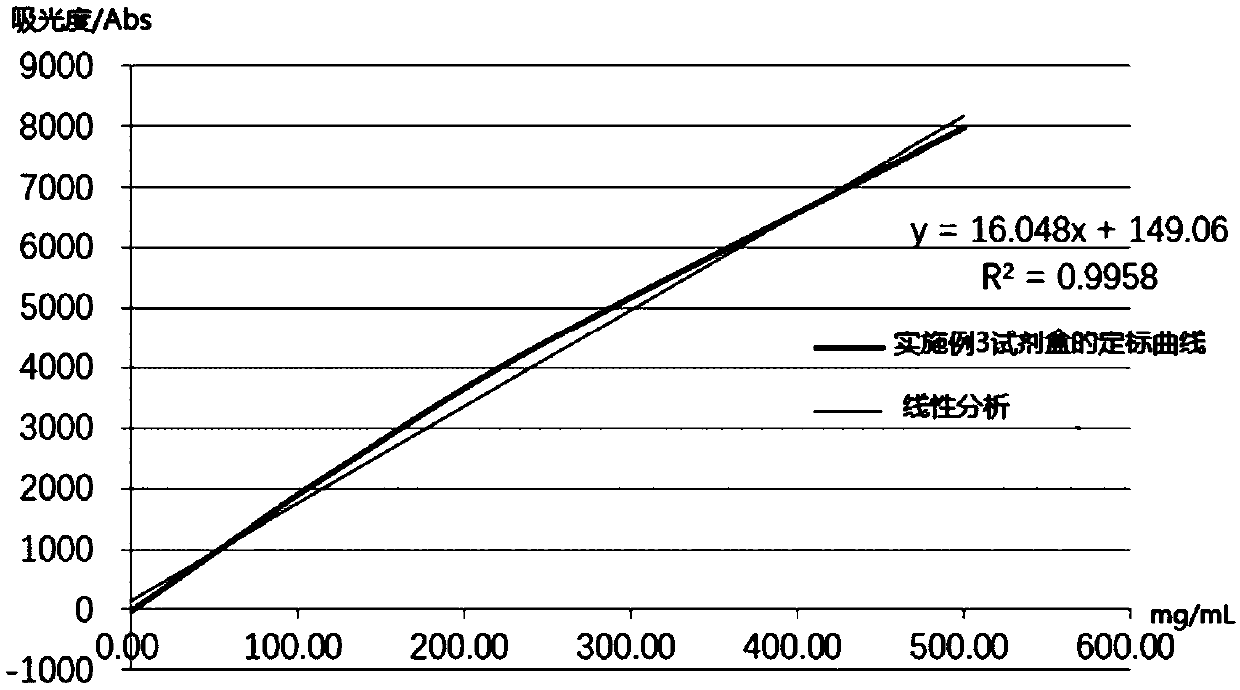
Embodiment 3
[0082] A human heparin-binding protein assay kit, comprising reagent 1, reagent 2 and human HBP calibrator with different concentration gradients; wherein,
[0083] Reagent 1 includes the following final concentrations of components: 20 mM PBS buffer (pH 7.2), 0.3% (w / v) BSA, 4% (w / v) PEG6000, 3% (w / v) Mw 10000 PVP, 20mM TECP, 50mM NH 4 Cl, 0.1% (w / v) Tween-80, 0.1% (w / v) ProClin300, 2mM EDTA;
[0084] Reagent 2 includes the components of the following final concentrations: carboxyl latex microspheres (average particle diameter is 309nm, microsphere final concentration is 0.01% (w / v)), 0.01% (w / v ) labeled human HBP monoclonal antibody, carboxyl latex microspheres labeled only with human HBP polyclonal antibody (average particle diameter is 83nm, final concentration of microspheres is 0.3% (w / v)), 0.035% (w / v) Labeled human HBP polyclonal antibody, 50mM TAPS buffer (pH8.0), 0.5% (w / v) gelatin, 0.5% (w / v) BSA, 2% (w / v) sucrose, 2% (w / v) Trehalose, 50 mM ammonium chloride, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com