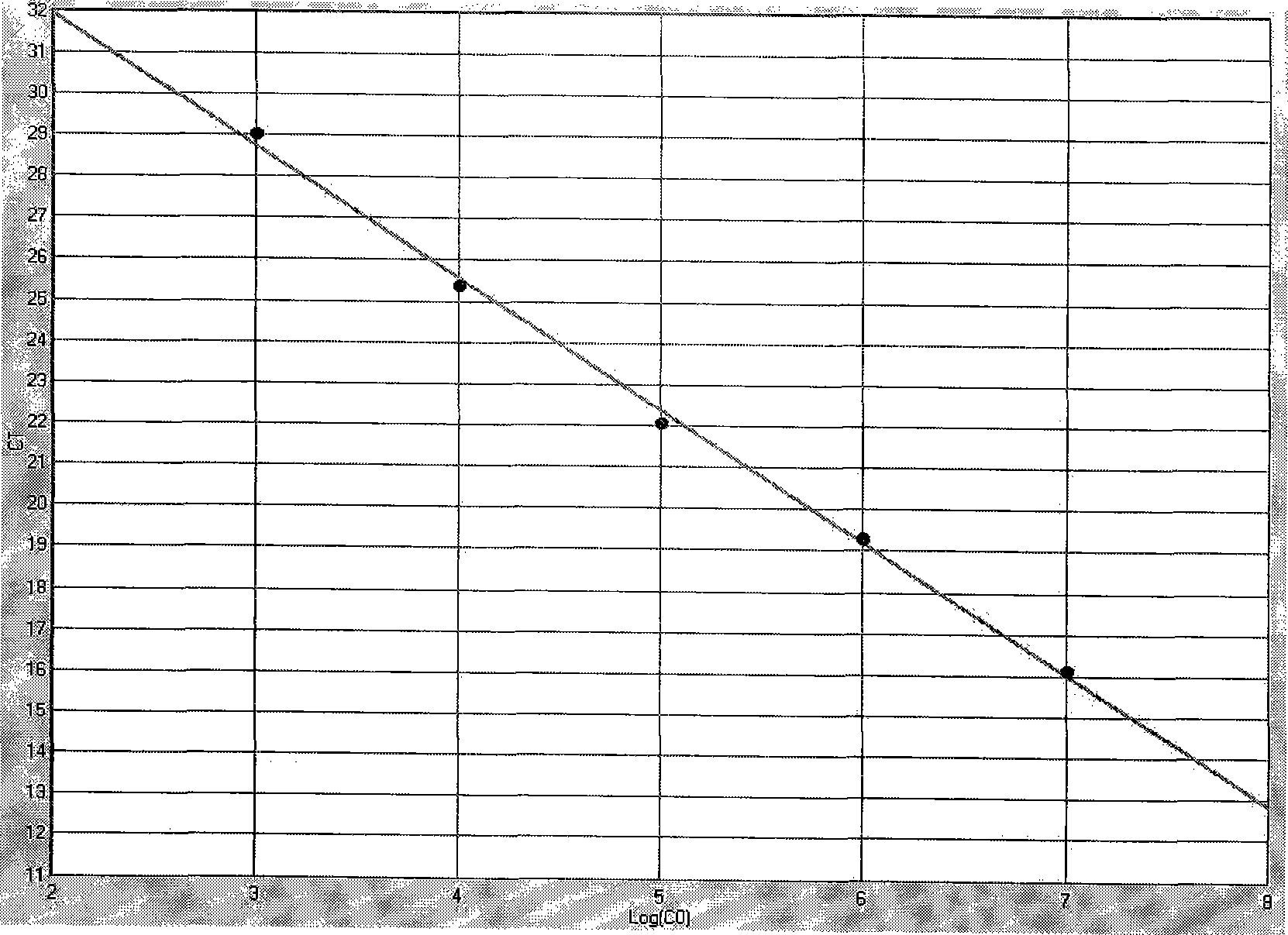
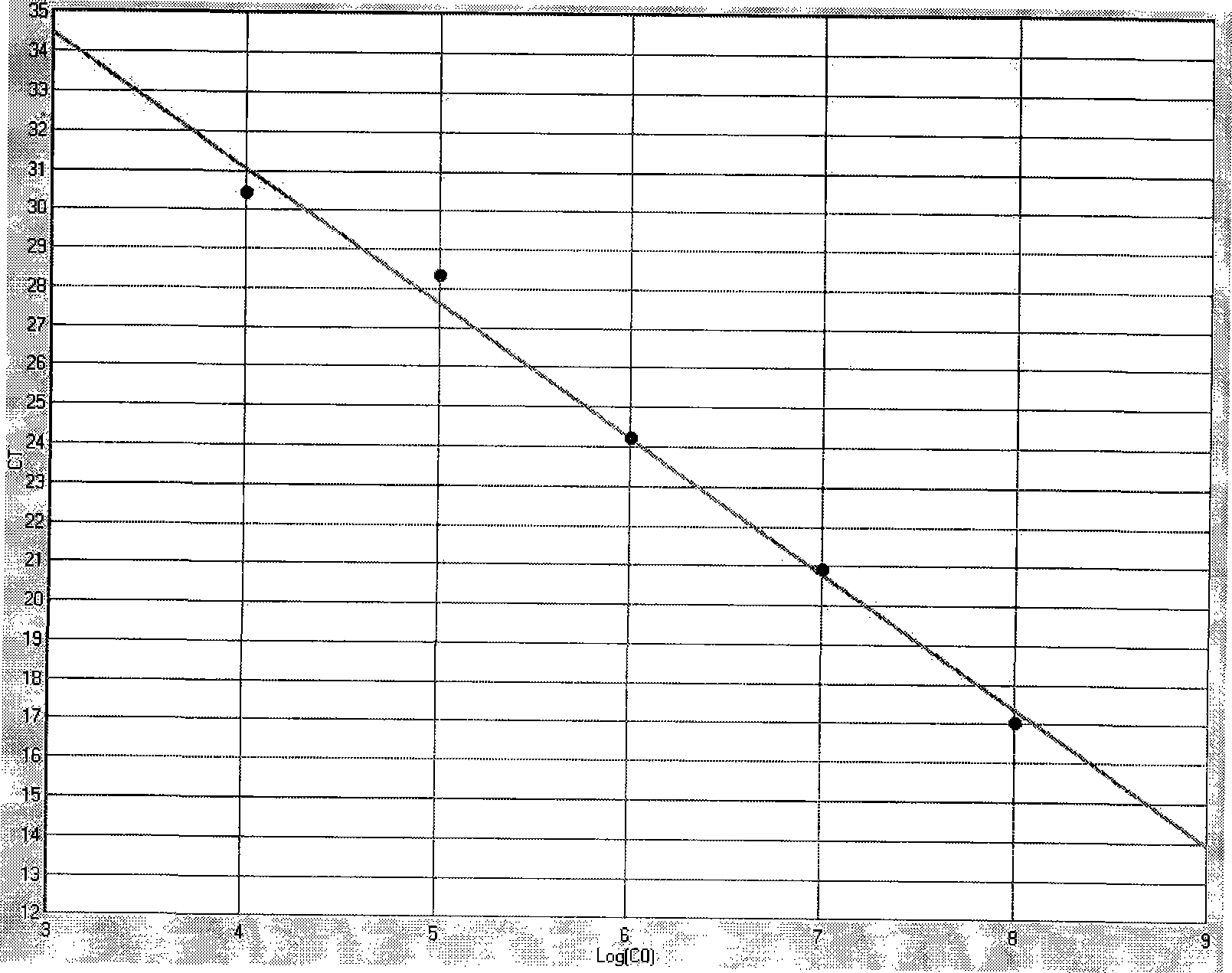
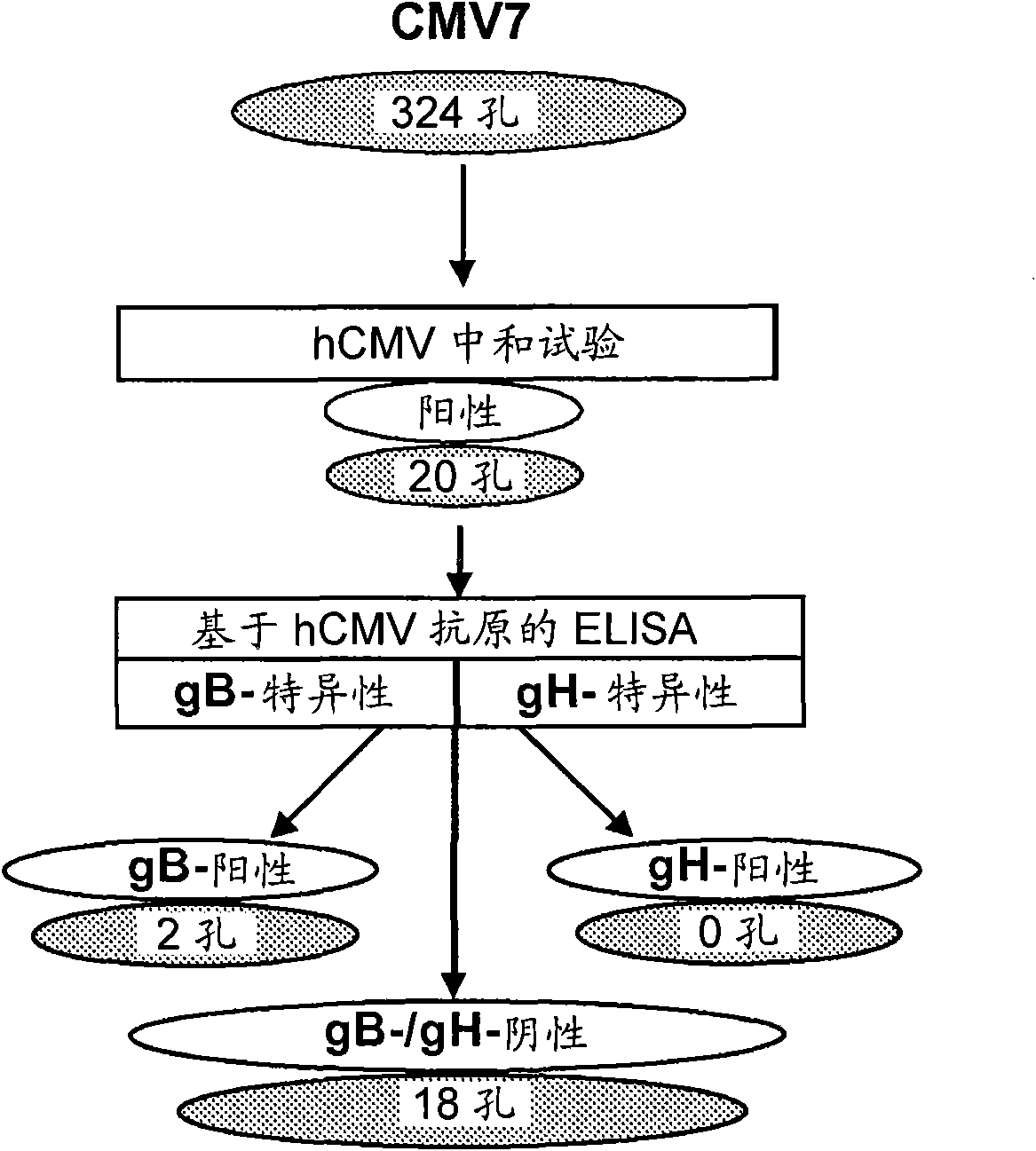
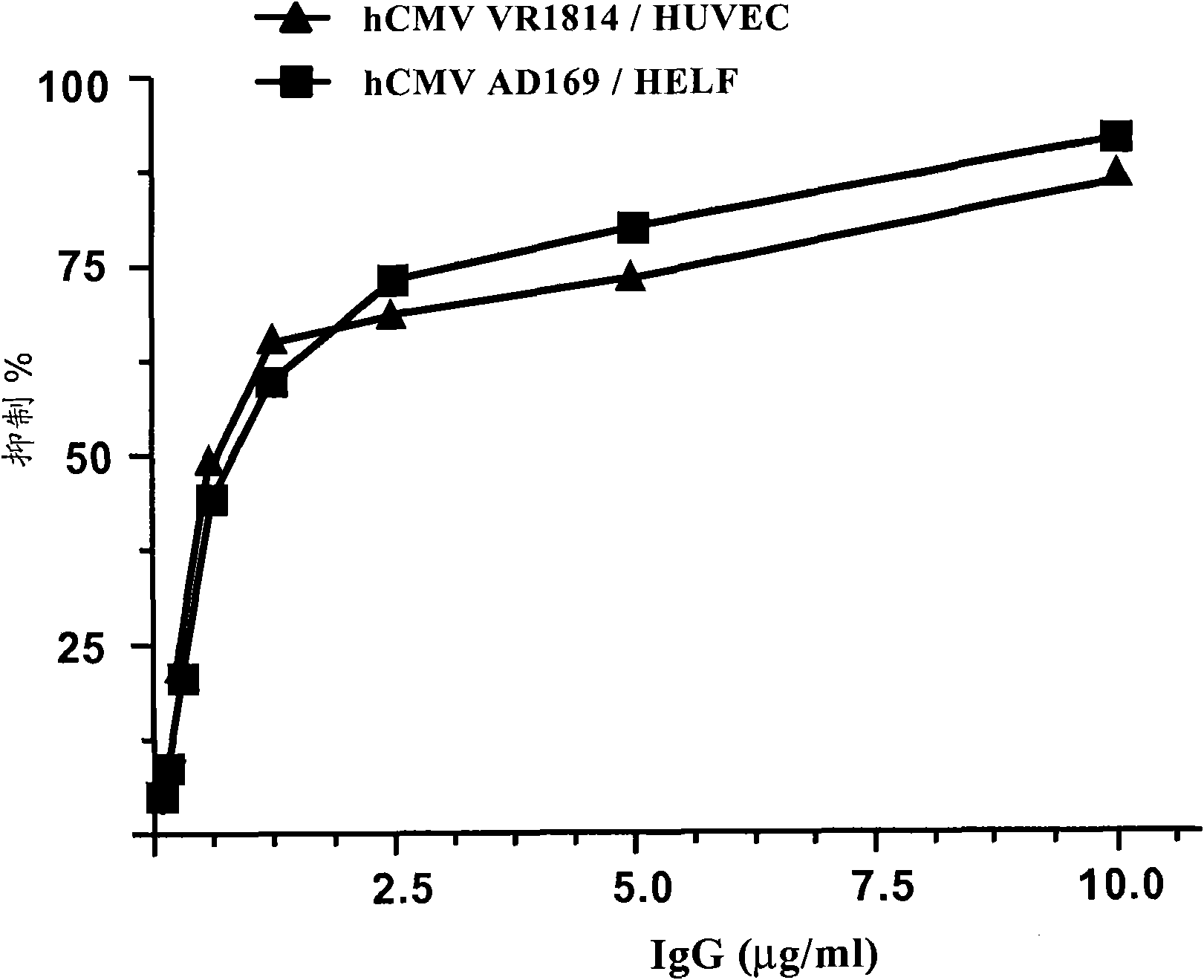
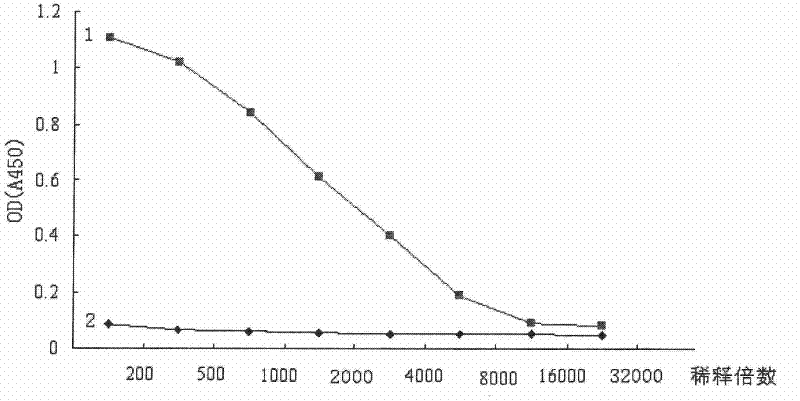
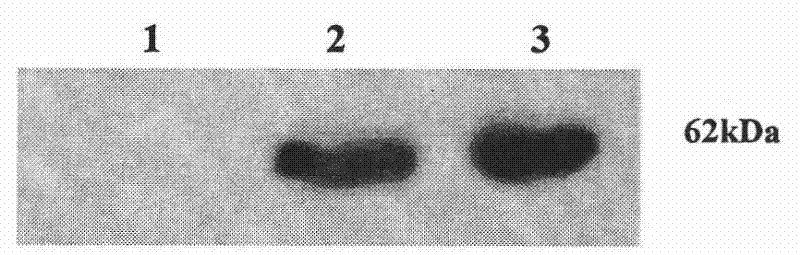
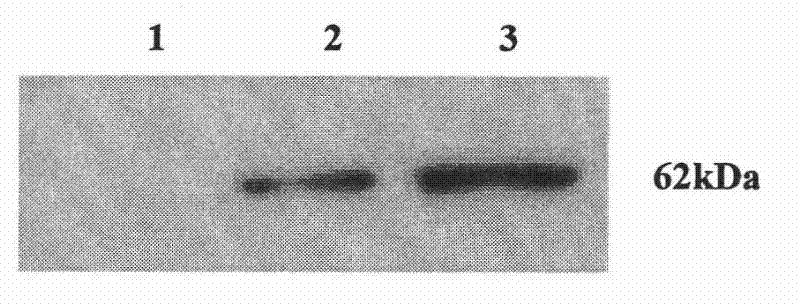
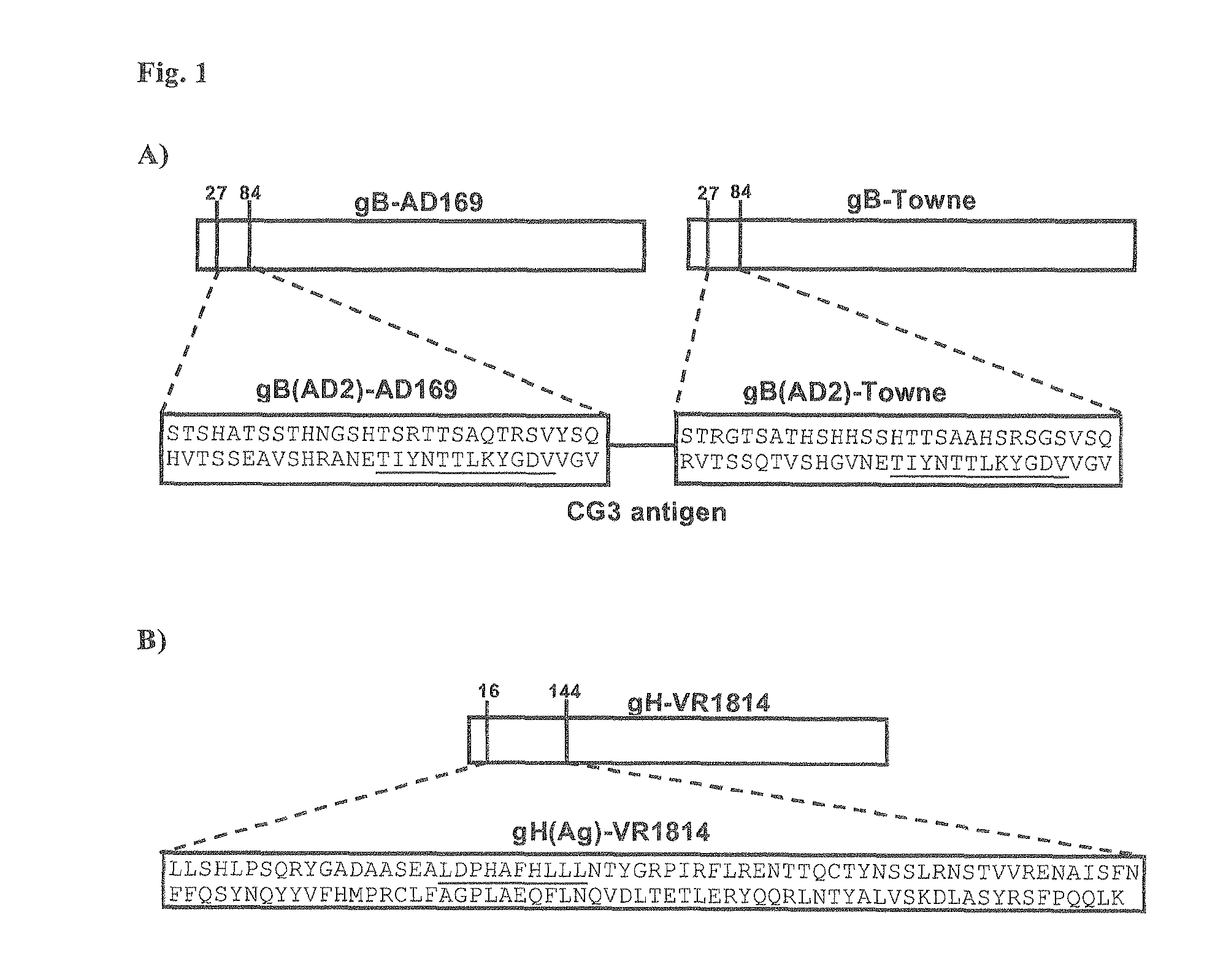
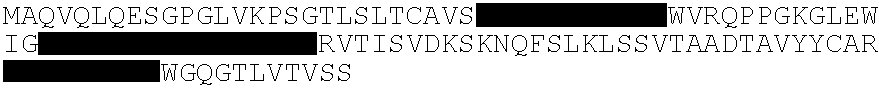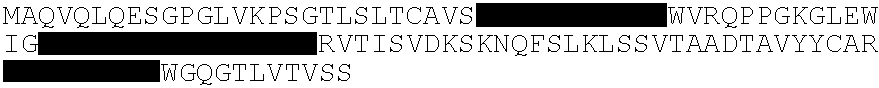Patents
Literature
52 results about "HCMV Infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human cytomegalovirus (HCMV) is a beta-herpesvirus that causes lifelong infection in humans. HCMV has a prevalence of 55-100% within the human population, depending on different socioeconomic and geographical factors.
Anti-human cytomegalovius antibodies
The present invention features a human antibody that specifically binds to human cytomegalovirus (HCMV), its encoding nucleic acid(s), and use of the antibody / nucleic acid(s) in treating HCMV infection.
Owner:DCB USA
Binding members for human cytomegalovirus
InactiveUS20130089559A1High affinityImprove performanceBacteriaPeptide/protein ingredientsHCMV InfectionBiological effect
The invention relates to binding members, especially antibody molecules, which may neutralise the biological effects of human cytomegalovirus (hCMV). The binding members may be useful for the treatment and prophylaxis of hCMV infection.
Owner:AGENUS INC
Expression monitoring for human cytomegalovirus (HCMV) infection
InactiveUS6936416B2Preventing and ameliorating disease symptomIncrease probabilitySugar derivativesMicrobiological testing/measurementHCMV InfectionHuman ARDS
Certain human genes have been found to be induced or repressed in host cells infected with HCMV. A large set of such genes has been identified. These have diagnostic use in determining the extent of tissue damage caused by the infection as well as in determining the stage of disease progression of the HCMV infection. Such genes are likely those involved in mediating the pathology of the infected tissues. Thus by identifying agents which are able to reverse the induction or repression of such genes, one can find candidate therapeutic agents for use in treating and or preventing HCMV-caused disease pathologies.
Owner:AFFYMETRIX INC
Antibodies against Human Cytimegalovirus (HCMV)
The present invention provides novel antibody sequences that bind human cytomegalovirus (hCMV) and neutralize hCMV infection. The novel sequences can be used for the medical management of hCMV infections, in particular for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of hCMV infections.
Owner:RIBOVAX BIOTECHNOLOGIES SA
Preparation method and application of anti-HCMV (human cytomegalovirus) Pp65 protein monoclonal antibody
The invention discloses a high-specificity and high-affinity anti-Pp65 monoclonal antibody which is generated by a hybridoma cell strain 8D6 of the anti-Pp65 monoclonal antibody, wherein the cell strain 8D6 is collected in China Center For Type Culture Collection (CCTCC) and has a collection number of CCTCC NO:C201476. The monoclonal antibody secreted by the hybridoma cell strain has good affinity and specificity, and the immunoreactivity and the specificity of the antibody are superior to those of domestic and imported monoclonal antibodies. The anti-Pp65 monoclonal antibody can serve as a key raw material for blood white cell immunofluorescent cytochemical diagnosis and immunofluorescent quantitative PCR (polymerase chain reaction) detection of HCMV (human cytomegalovirus) infection, also can serve as a key raw material for detecting HCMV by using a double antibody sandwich method, has important meaning for establishment of a quick and sensitive clinical diagnosis reagent to HCMV infection, and has important application prospects.
Owner:HUNAN NORMAL UNIVERSITY
Building method of human spongioblastoma cell SCID mouse brain transplantation tumor model
InactiveCN108186681AReflect the actual infection statusGrowth toleranceMammal material medical ingredientsVeterinary instrumentsSingle cell suspensionHCMV Infection
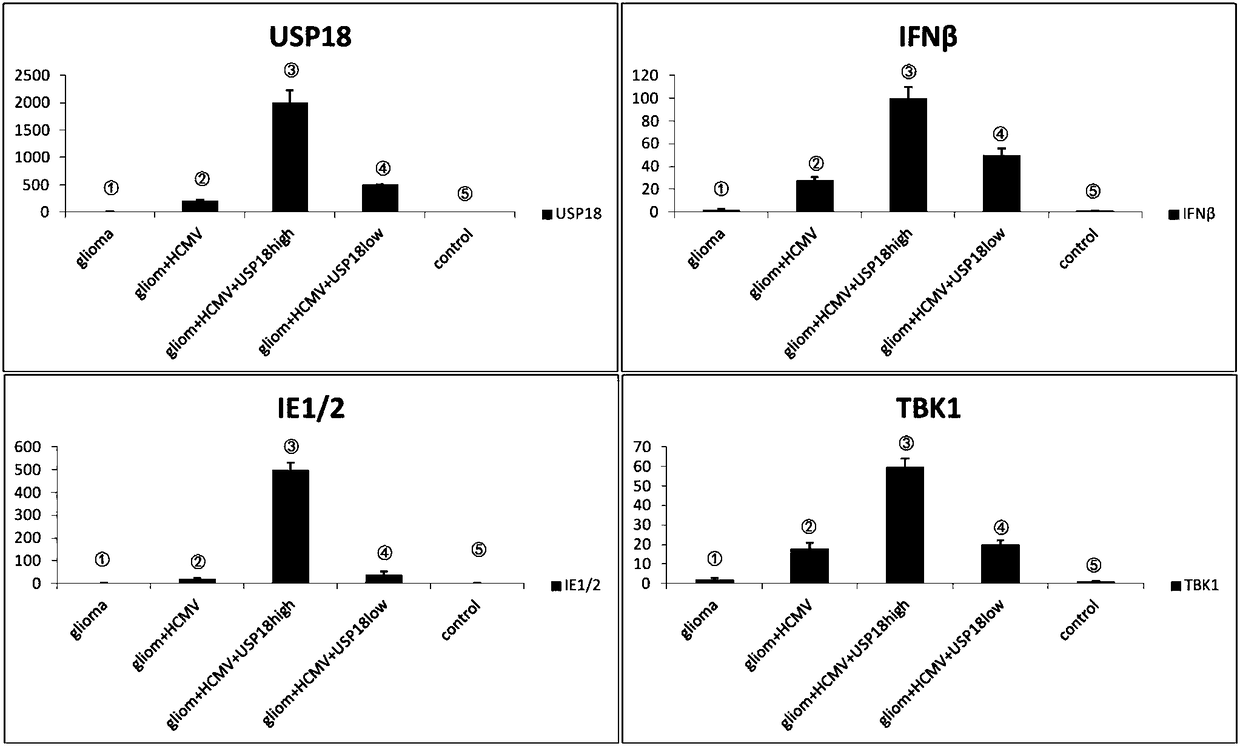
The invention discloses a building method of a human spongioblastoma cell SCID mouse brain transplantation tumor model. The method comprises the following steps of preparing primary spongioblastoma cells; selecting male SCID mice; dividing the mice into five groups (the first group of mice are inoculated with primary generation spongioblastoma cells in the brain; the second group of mice are inoculated with primary generation spongioblastoma cells in the brain and are infected with HCMV; the third group of mice are inoculated with primary spongioblastoma cells, and are infected with HCMV, andUSP18 high expression adenovirus carriers are used for processing the group; the fourth group of mice are inoculated with primary generation spongioblastoma cells in the brain and are infected with HCMV, interference adenovirus carrier USP18 small interference adenovirus carriers USP18 are used for treating the group of mice; the fifth group of mice is a normal control group); determining a targeted point; performing aseptic inoculation of single-cell suspension; during experiment termination, cutting the head; taking the brain; identifying whether the building of the human spongioblastoma cell SCID mouse brain transplantation tumor model is successful or not. The tumor model can better embody the practical infection state of the HCMV infected human spongioblastoma.
Owner:QINGDAO UNIV
Preparation method of human macrocell virus pp65 protein vaccine
A human cytomegalovirus pp65 protein vaccine for preventing and blocking primary HCMV infection is prepared through extracting HCMV DNA, amplifying it to obtain the gene fragment pp65(1006-152 / nt), cloning it to the prokaryotic expression carrier PET100-TOPO, exogenous target gene expression in E.coli BL21 cell, extracting target fragment protein pp65, and purifying.
Owner:王明丽
Method for detection of infection with human cytomegalovirus
InactiveCN103003694AHigh detection sensitivityAccurate detectionVirus peptidesDepsipeptidesEscherichia coliAntigen
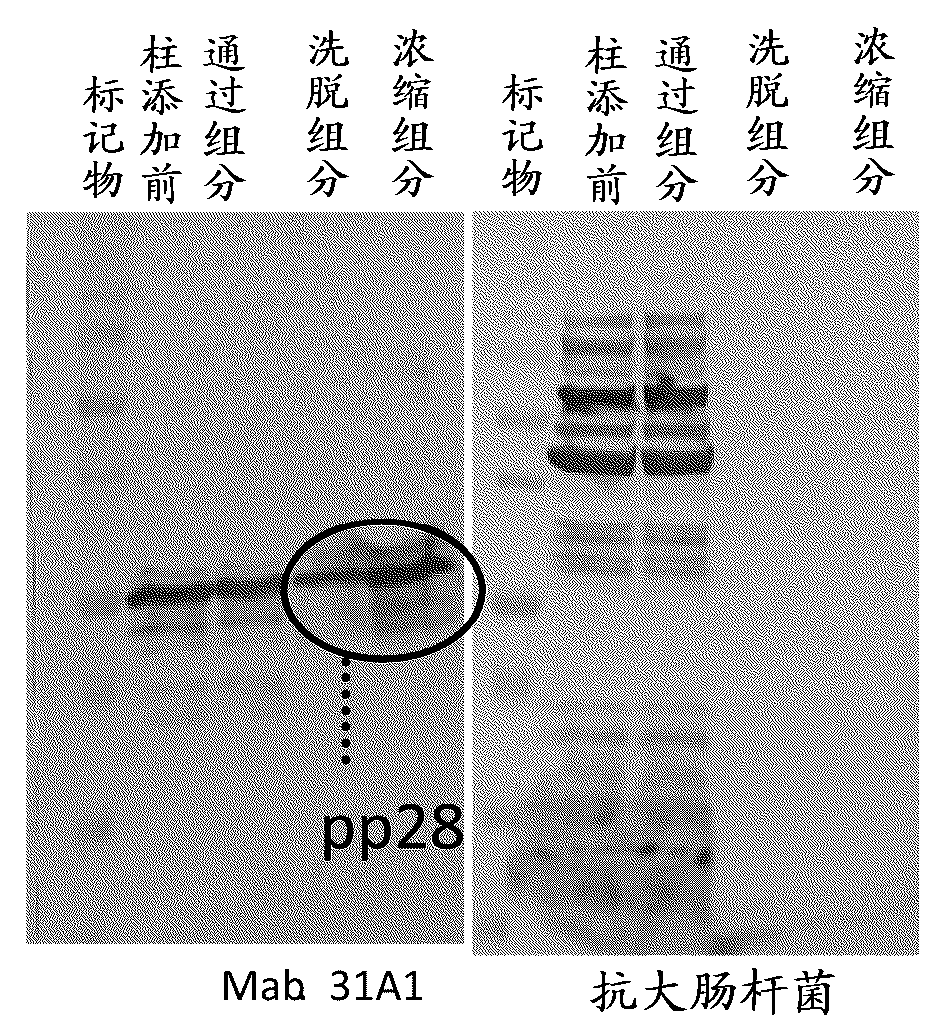
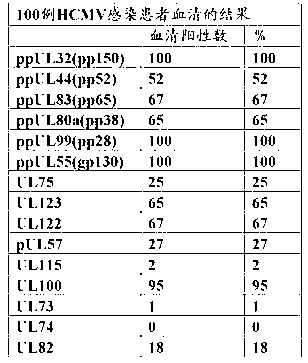
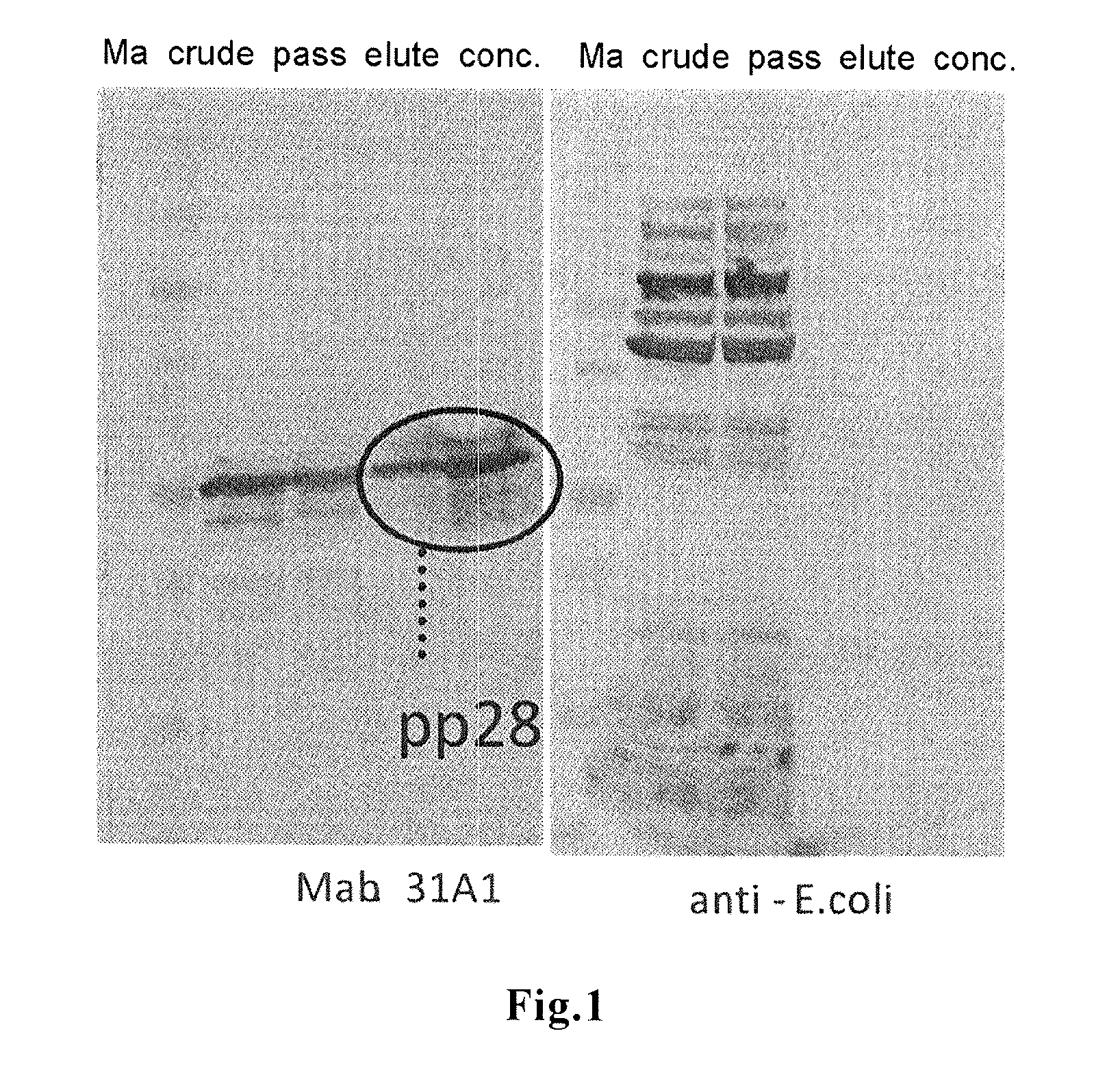
Disclosed is a novel and highly practically available means for detecting the infection with an HCMV with high sensitivity. A full-length protein of each of 15 kinds of HCMV proteins is synthesized, and the reactivity of the full-length protein with serum collected from each of HCMV infection patients is extensively examined. As a result, it is found that all of infection patients can be detected completely when a full-length pp28 protein is used as an antigen. The full-length pp28 protein can be synthesized in a large amount in the form of a recombinant protein and purified using Escherichia coli, and can be used for commercial purposes as an antigen for use in the test on HCMVs.
Owner:FUJIREBIO CO LTD
Method for detection of infection with human cytomegalovirus
InactiveUS20130109010A1High detection sensitivitySurely detectedPeptide/protein ingredientsMicrobiological testing/measurementEscherichia coliAntigen
A novel means by which HCMV infection can be detected with a high sensitivity and whose practicality is high is disclosed. The present inventors synthesized as many as 15 kinds of HCMV proteins in their full length forms, and intensively studied their reactivities with sera from HCMV-infected patients to find that all the infected patients can be detected without fail when using the pp28 full length protein as an antigen. The pp28 full length protein can be synthesized and purified as a recombinant protein in a large scale by using Escherichia coli, and can be commercially used as an antigen for HCMV tests.
Owner:FUJIREBIO CO LTD
Typing method for detection of HCMV gBn by real-time fluorescent quantitative PCR
InactiveCN101397591AAvoid pollutionStrong specificityMicrobiological testing/measurementFluorescenceTyping methods
The invention discloses a method for detecting HCMV gBn classification by real-time fluorescence quantitative PCR. The method comprises the following steps: a fluorescent probe is added in a PCR reaction system, the PCR process is monitored in real time by the fluorescent signal accumulation and quantitative and qualitative analysis is carried out to determine the type of HCMV gBn. The invention is closed reaction without PCR post treatment, avoids pollution, has strong specificity and high sensitivity, adopts logarithmic phase analysis and abandons destination data and has accurate quantification; the real-time fluorescence quantitative PCR technology can carry out classification and quantification on HCMV gB; the on-line real-time detection by instruments has intuitive result and avoids artificial judgment; double-detection or multi-detection by one tube can be realized; and the operation is safe and the time is shortened. The common PCR needs about 1 to 2 days, while the method of the invention generally needs only 3 to 4 hours, thus improving the efficiency; the invention can be used for clinically detecting gBn classification of HCMV-infected crowd, predicting the prognosis, observing HCMV activity infection and guiding clinical treatment.
Owner:ZHEJIANG UNIV
Antibodies against human cytomegalovirus (hcmv)
InactiveCN101627115AOrganic active ingredientsPeptide/protein ingredientsHCMV InfectionTherapeutic treatment
The present invention provides novel antibody sequences that bind human cytomegalovirus (hCMV) and neutralize hCMV infection. The novel sequences can be used for the medical management of hCMV infections, in particular for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of hCMV infections.
Owner:RIBOVAX BIOTECHNOLOGIES SA
Antibodies against human cytimegalovirus (HCMV)
The present invention provides novel antibody sequences that bind human cytomegalovirus (hCMV) and neutralize hCMV infection. The novel sequences can be used for the medical management of hCMV infections, in particular for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of hCMV infections.
Owner:RIBOVAX BIOTECHNOLOGIES SA
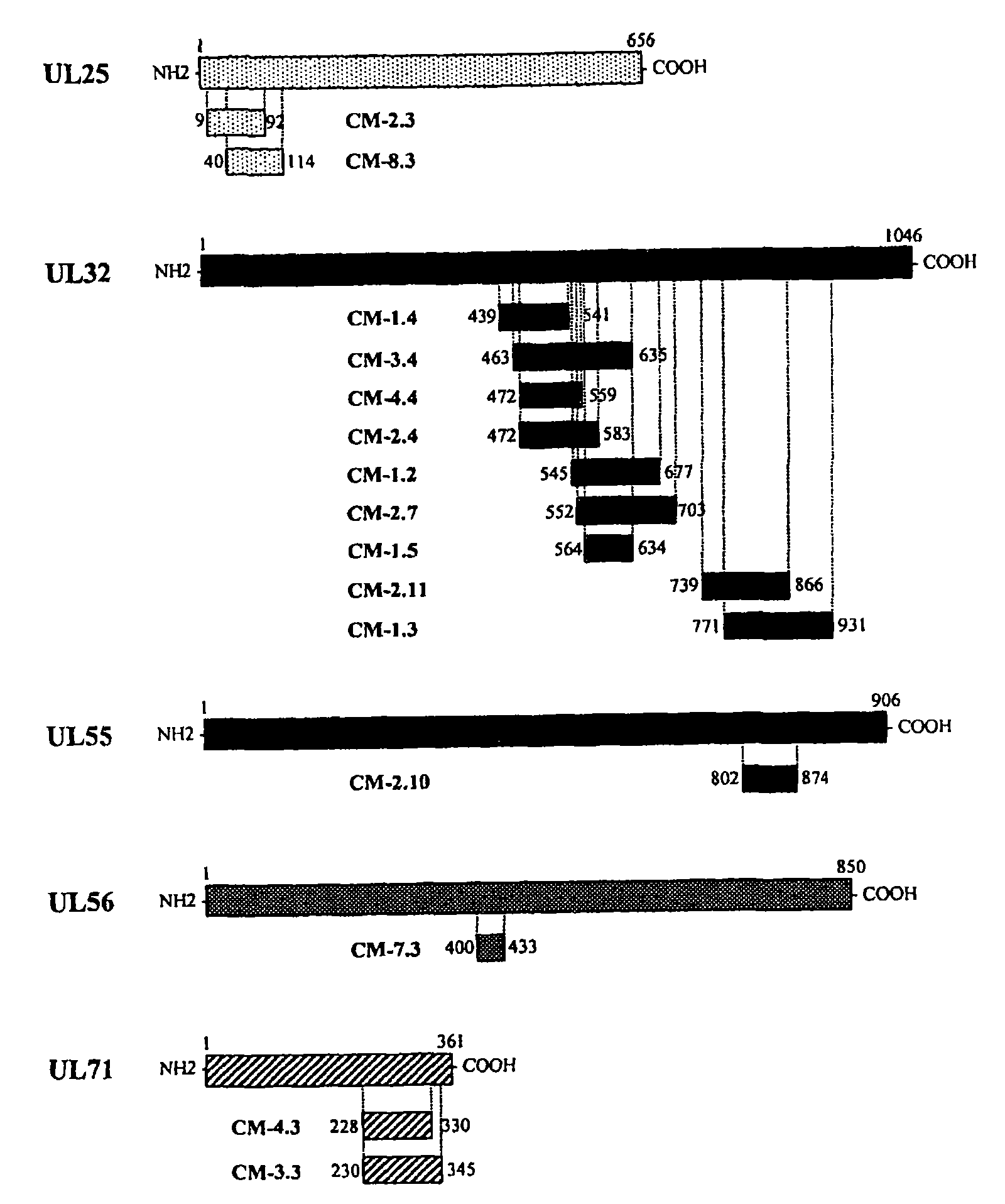
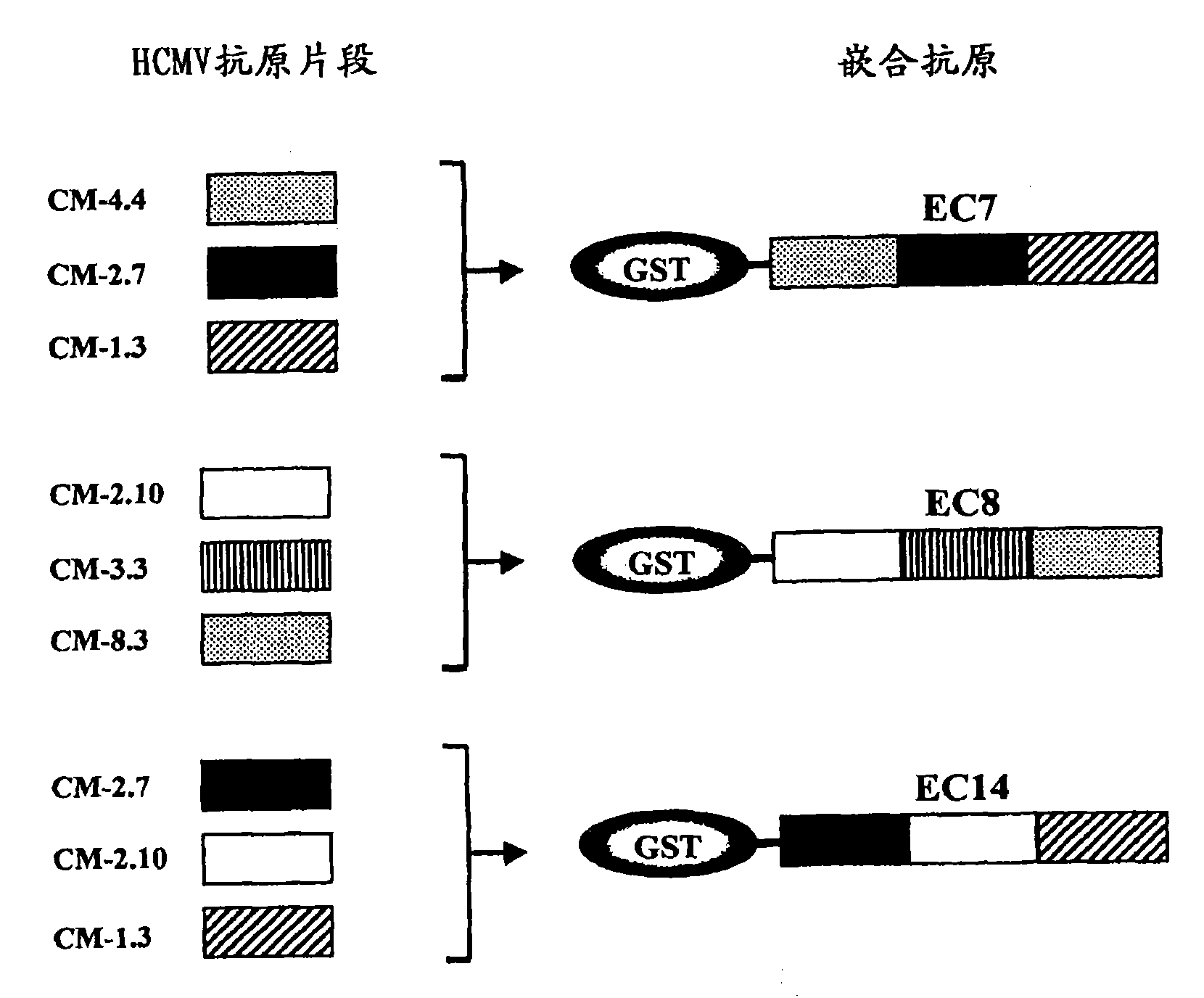
Recombinant antigens of human cytomegalovirus (HCMV)
The invention described herein relates to a method for identifying the antigenic regions of HCMV proteins involved in the human B-cell response to HCMV infection, for combining such antigenic regions in the form of chimeric fusion products, and their use as diagnostic and immunogenic agents.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Novel and highly-sensitive detection method for latent human cytomegalovirus (HCMV) in cell
InactiveCN103627822AHigh homologyMicrobiological testing/measurementPeripheral blood mononuclear cellHCMV Infection
The invention provides a novel and highly-sensitive detection method for human cytomegalovirus (HCMV). The method represents a nested quantitative PCR (polymerase chain reaction) technique which can detect both free HCMV and HCMV that infects a cell, and is characterized in that nucleic acid is extracted from human peripheral blood mononuclear cells (PBMCs), and a first round of PCR amplification is carried out through specific outer upstream and downstream primers; with the products of the first round of PCR amplification serving as a template, a second round of quantitative PCR amplification is carried out through specific inner upstream and downstream primers. By adopting the detection method provided by the invention, the HCMV detection sensitivity can reach 8 copies per microliter. Compared with the ELISA (enzyme-linked immunoabsorbent assay) method, compared with the ELISA method, the method adopting the PCR method for the detection of the latent HCMV infection has the advantages of rapidness, simplicity, high sensitivity, strong specificity, good stability and the like, and is a clinical method for early detection and diagnosis.
Owner:WENZHOU MEDICAL UNIV
Method for detecting harm degree of postoperative cytomegalovirus infection of kidney transplantation patient
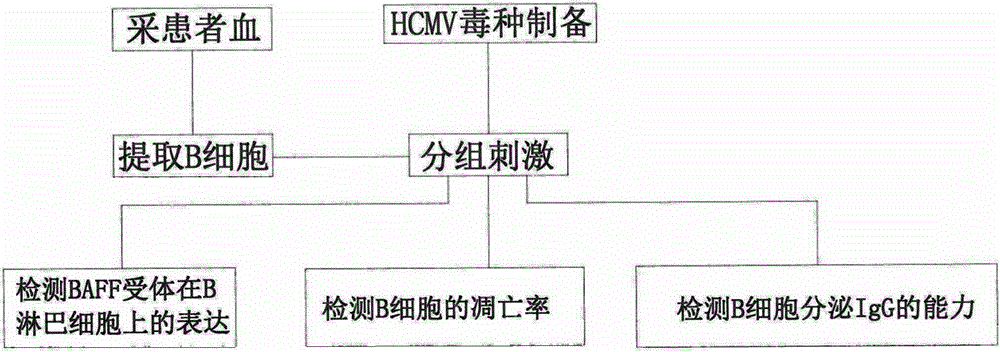
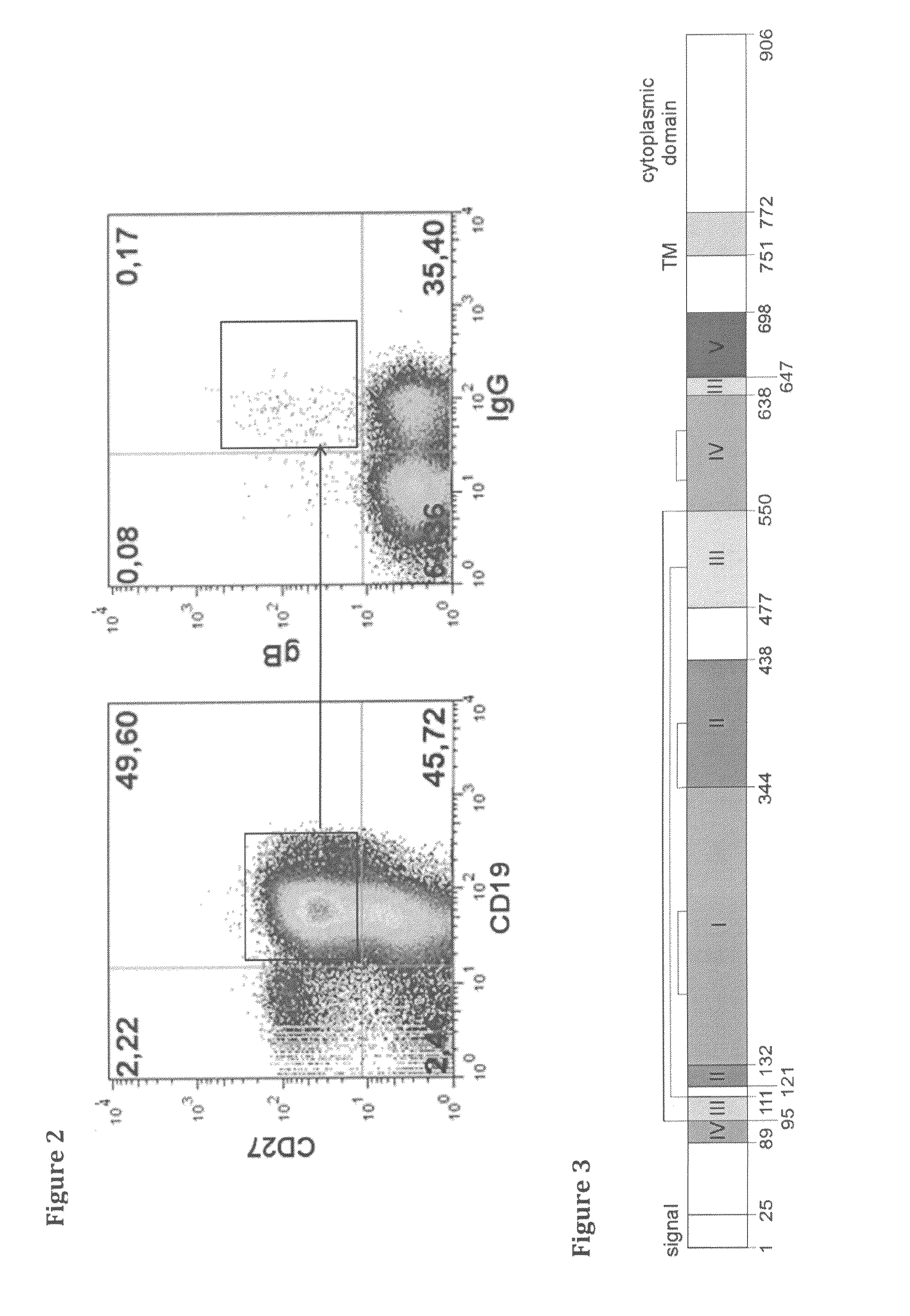
PendingCN105296588AReduce the risk of HCMV infectionMicrobiological testing/measurementBlood/immune system cellsApoptosisHCMV Infection
The invention relates to a method for detecting harm degree of postoperative cytomegalovirus infection of a kidney transplantation patient. The invention includes the following steps: a, collecting blood; b, acquiring and analyzing CD19+B lymphocytes; c, preparing cytomegalovirus seeds and quantifying; d, culturing the CD19+B lymphocytes in vitro, and using HCMV to directly and indirectly stimulate the CD19+B lymphocytes; e, performing flow detection on expression of a BAFF receptor on the CD19+B lymphocytes; f, detecting apoptosis rate of the CD19+B lymphocytes; g, detecting IgG secretion capability of the CD19+B lymphocytes. By the method, reaction, to HCMV stimulation, of the B lymphocytes of the patient can be analyzed. The method has important value in evaluating chronic rejection which is indirect effect caused by HCMV infection of the kidney transplantation patient.
Owner:THE FIRST PEOPLES HOSPITAL OF CHANGZHOU
Compositions and methods for treatment of cytomegalovirus
PendingCN107875382ASsRNA viruses negative-senseAntibody mimetics/scaffoldsEpitopeVirus-like particle
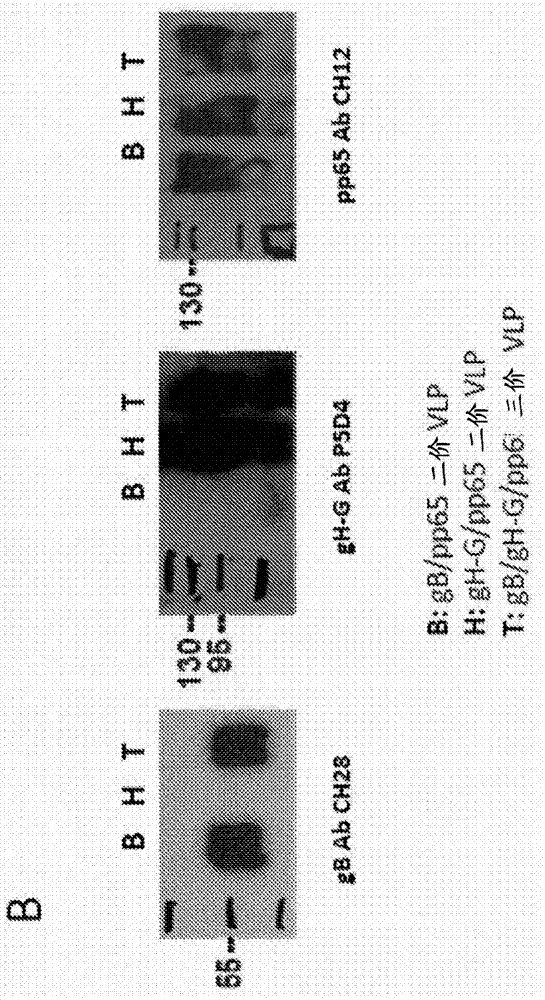
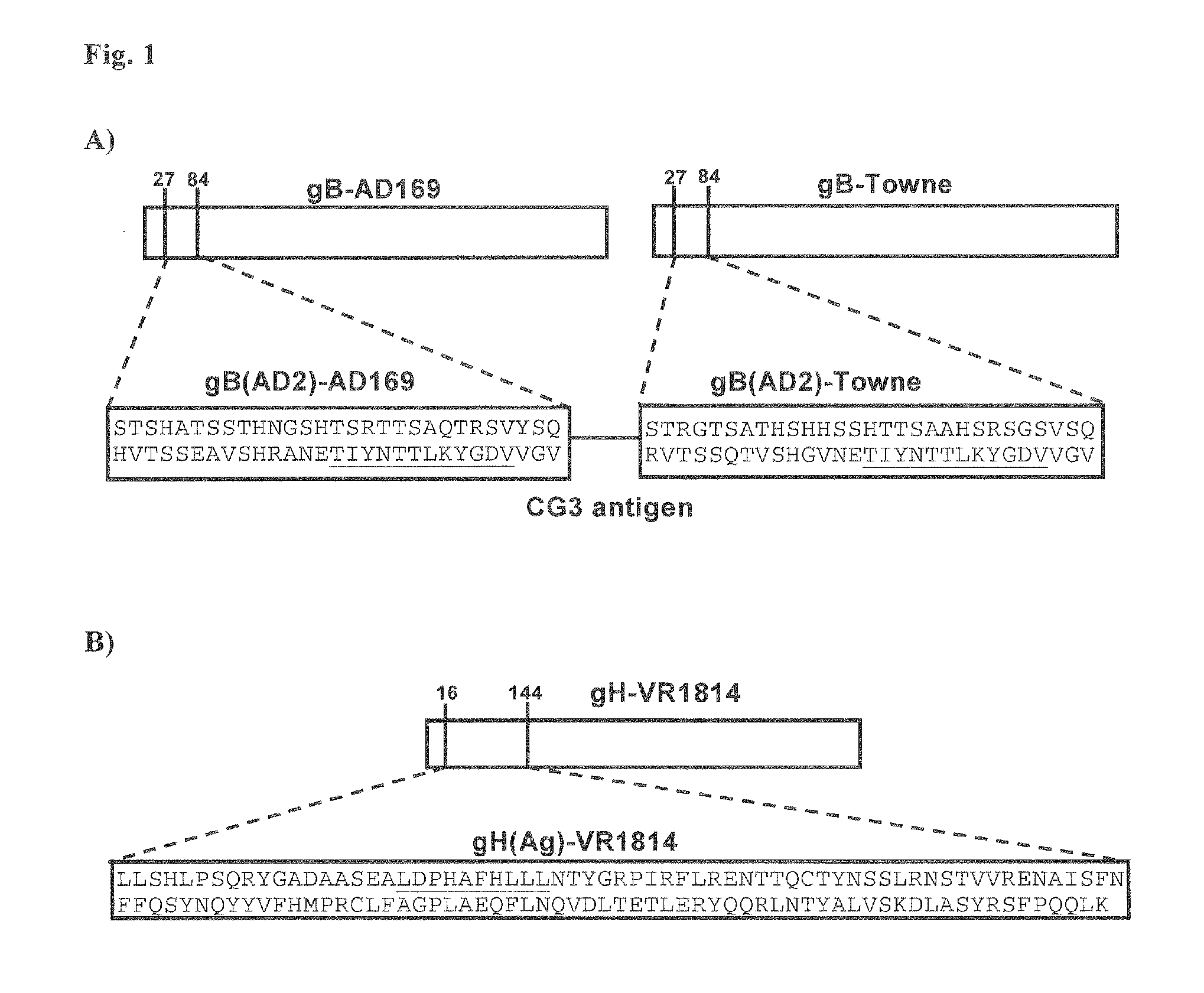
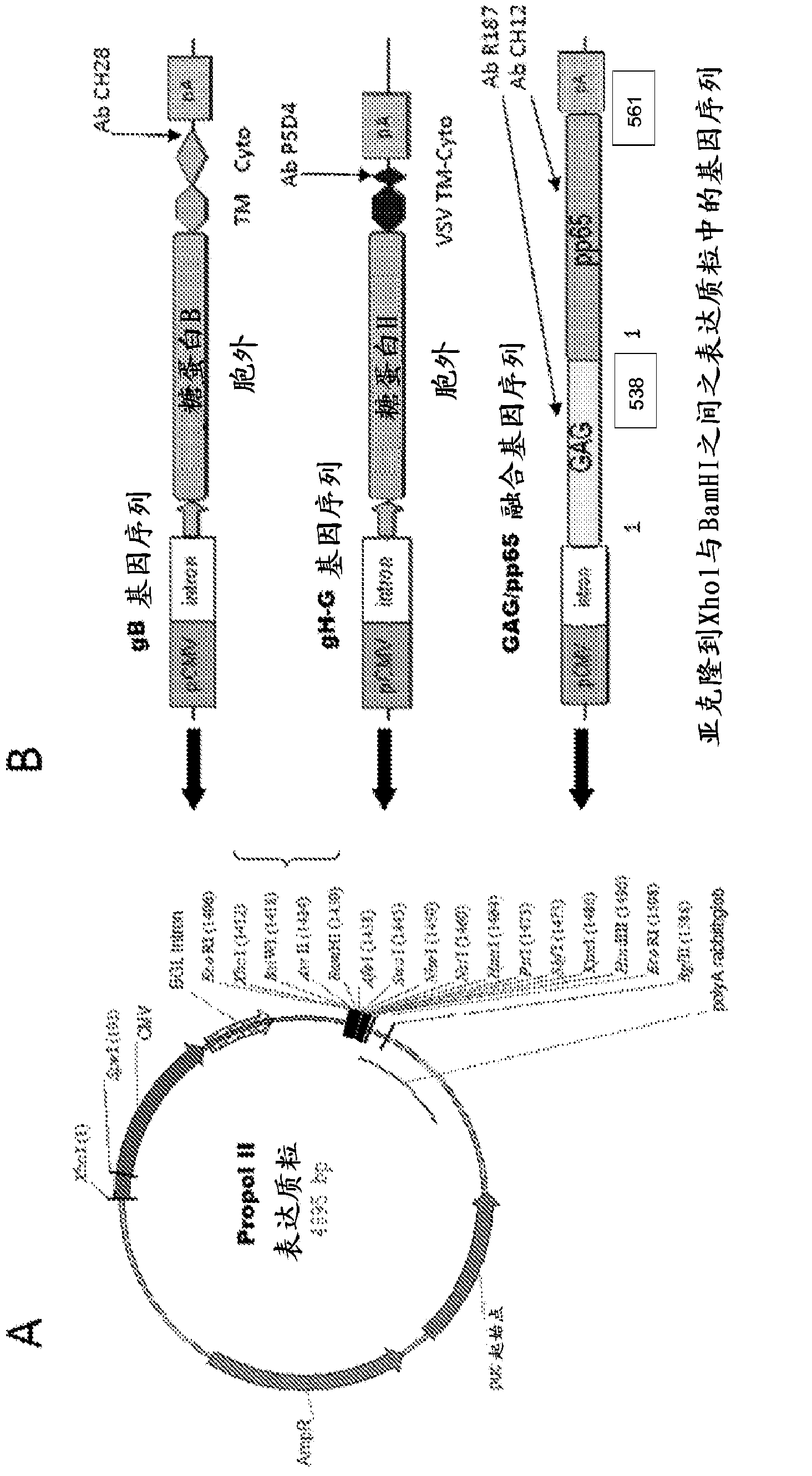
The present disclosure provides compositions and methods useful for treating HCMV infection. As described herein, the compositions and methods are based on development of immunogenic compositions thatinclude virus-like particles (VLPs) which comprise one or more Moloney Murine leukemia virus (MMLV) core proteins and include one or more HCMV epitopes, such as, for example, from HCMV envelope glycoproteins gB and / or gH and / or tegument protein pp65. Among other things, the present invention encompasses the recognition that a combination of antigens (e.g., envelope glycoproteins and structural proteins) can lead to beneficial immune responses, for example that include both a humoral response (e.g., production of neutralizing antibodies) and a cellular response (e.g., T-cell activation).
Owner:VARIATION BIOTECHNOLOGIES INC
Antibodies Against Human Cytomegalovirus (HCMV)
InactiveUS20110171233A1Organic active ingredientsPeptide/protein ingredientsHCMV InfectionTherapeutic treatment
The present invention provides novel antibodies sequences that bind human cytomegalovirus (hCMV) and neutralize hCMV infection. The novel sequences can be used for the medical management of hCMV infections, in particular for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of hCMV infections.
Owner:RIBOVAX BIOTECHNOLOGIES SA
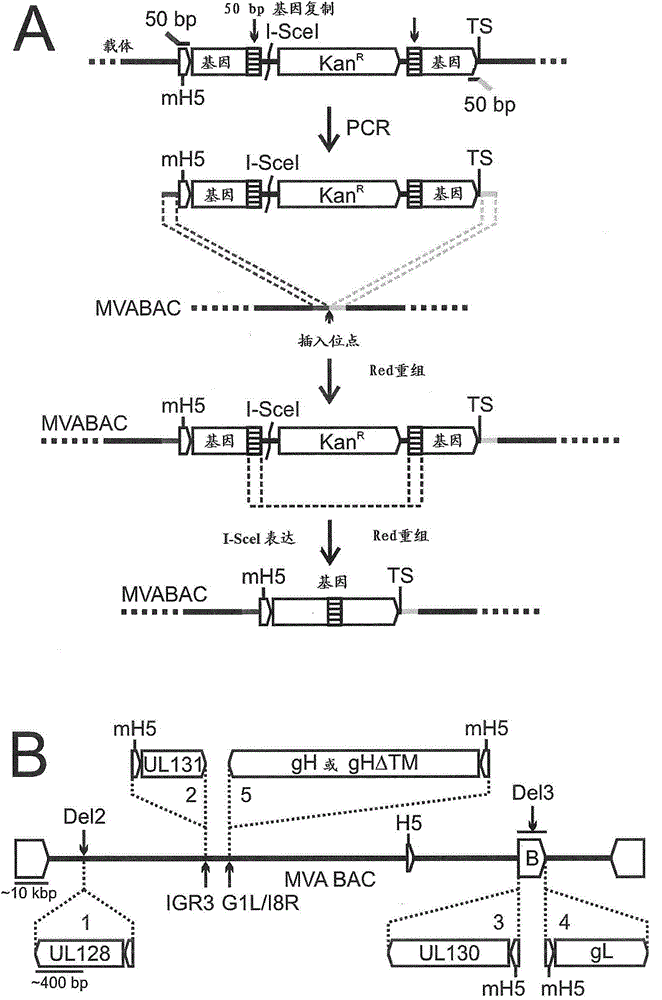
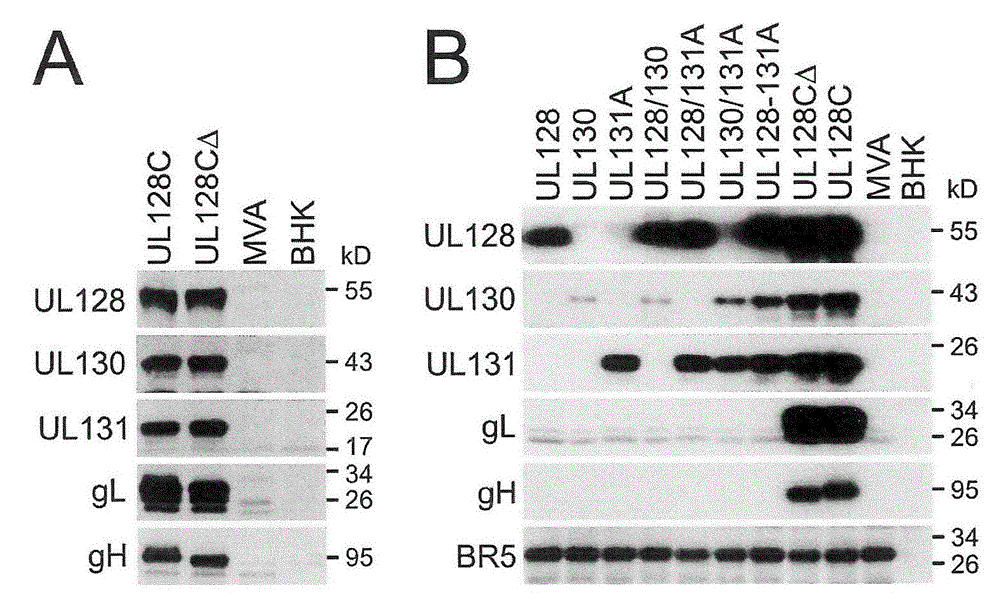
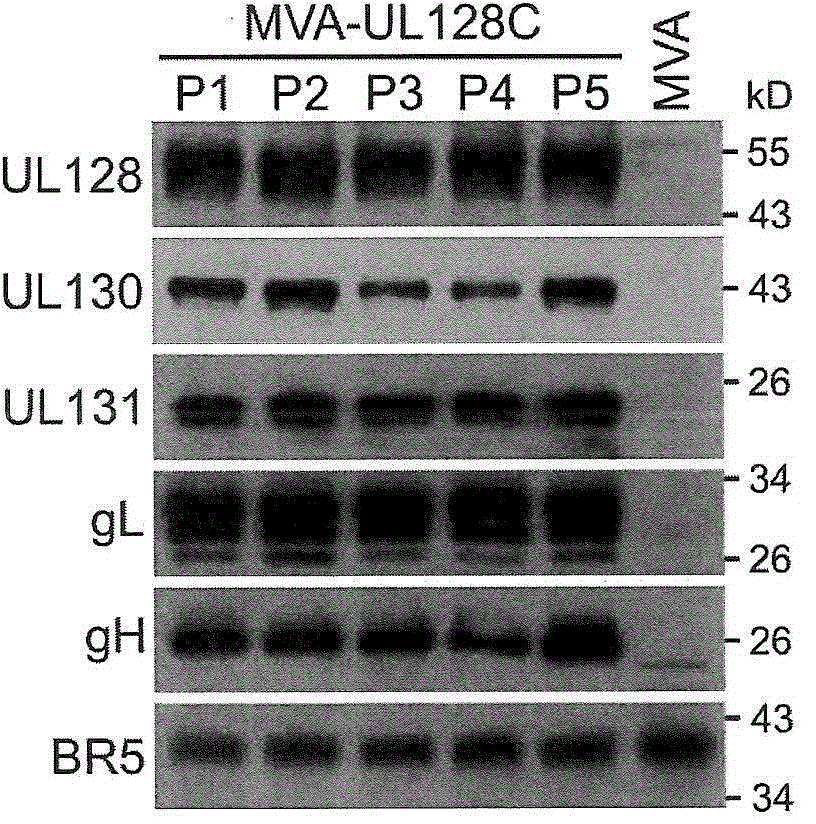
An MVA vaccine for delivery of a UL128 complex and preventing CMV infection
In one embodiment, an expression system for expressing a UL128 complex is provided herein. The expression system may include a bacterial artificial chromosome (BAC) construct, wherein the BAC construct comprises a viral vector inserted with a set of DNA sequences that encode a UL128 complex. In another embodiment, a vaccine composition for preventing HCMV infection is provided. The vaccine composition may include a viral or bacterial vector capable of expressing a UL128 complex and a pharmaceutically acceptable carrier, adjuvant, additive or combination thereof or additional vector expressing a protein adjuvant. The viral vector may be an MVA and the UL128 complex includes five HCMV proteins or antigenic fragments thereof: UL128, UL130, UL131A, gL, and gH. In some embodiments, the viral vector is further inserted with one or more additional DNA sequences that encode one or more additional HCMVHCMV proteins or antigenic fragments thereof such as pp65, gB or both, or such as gM / gN or gO.
Owner:CITY OF HOPE
Anti-human cytomegalovirus antibodies
Owner:DCB USA
Recombinant antigens of human cytomegalovirus (HCMV)
InactiveUS20100068229A1Improve performanceOrganic active ingredientsVirusesHCMV InfectionImmunogenicity
The invention described herein relates to a method for identifying the antigenic regions of HCMV proteins involved in the human B-cell response to HCMV infection, for combining such antigenic regions in the form of chimeric fusion products, and their use as diagnostic and immunogenic agents.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Preparation method of Anji white tea polysaccharides and novel use of the polysaccharides against human cytomegalovirus
ActiveCN109535270AInhibition of lesionsLow autotoxicityAntiviralsBulk chemical productionCytopathic effectHCMV Infection
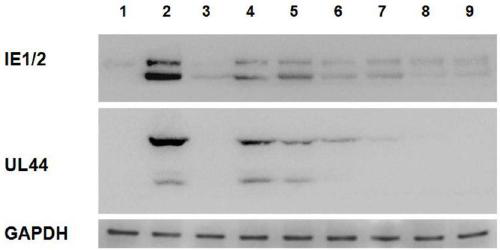
The invention belongs to the field of biotechnology and relates to preparation of Anji white tea polysaccharides and use of the polysaccharides against a human cytomegalovirus (HCMV). The Anji white tea polysaccharides prepared by supercritical CO2 extraction have obvious anti-HCMV effect, and are characterized by significantly suppressing expression of HCMV characteristic genes IE1 / 2 and UL44 inits in vitro host cells that are human embryonic lung fibroblast, effectively suppressing DNA replication of HCMV, and significantly improving the cytopathic effect caused by HCMV infection. The Anjiwhite tea polysaccharides have low toxicity, and have the application prospect of preparing anti-HCMV drugs.
Owner:ZHEJIANG HOSPITAL
Binding members for human cytomegalovirus
Owner:AGENUS INC
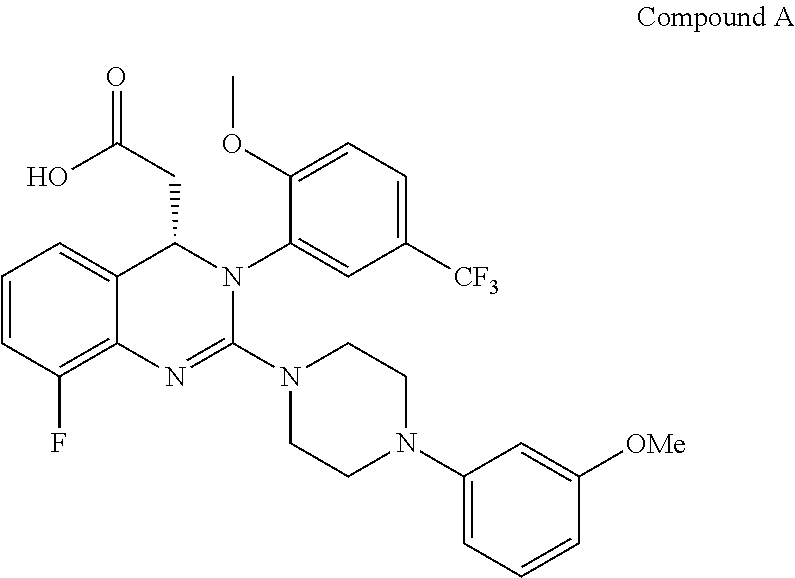
Process for making substituted quinazoline compounds
ActiveUS20160311781A1Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHCMV InfectionQuinazoline
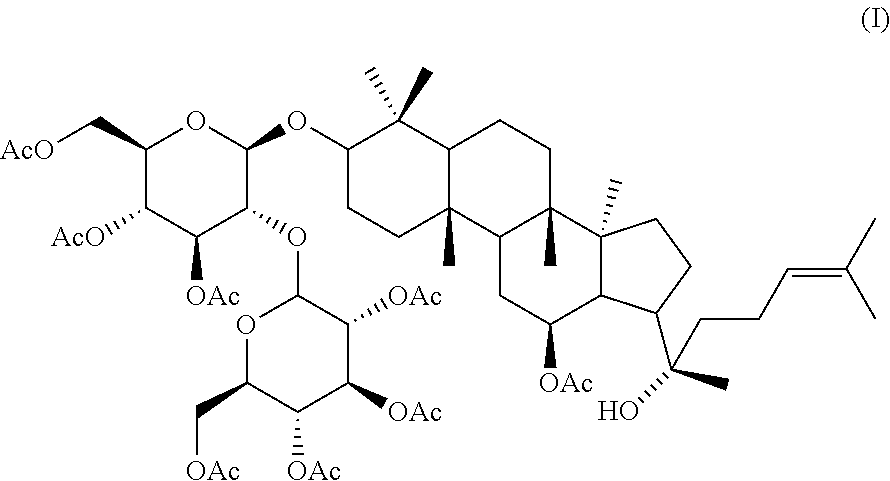
The present invention is directed to a process for making Substituted Quinazoline Compounds of formula (I): which are useful for the treatment and prophylaxis of HCMV infection. The present invention is also directed to compounds that are useful as synthetic intermediates for making the compounds of formula (I).
Owner:AICURIS ANTI INFECTIVE CURES +1
A kind of human cytomegalovirus ul49 gene antigen peptide, its antibody and application
InactiveCN101580537BHigh purityChemical synthesis is easyMicroorganismsImmunoglobulins against virusesChemical synthesisAntigen
The present invention discloses a human cytomegalovirus UL49 gene antigenic peptide, its antibody and application. The antigenic peptide has the amino acid sequence shown in SEQ ID No.1, and its nucleotide sequence is shown in SEQ ID No.2. The antigenic peptide of the present invention is a 12-short peptide, which is easy to chemically synthesize and has high product purity. The antibody prepared by using the antigenic peptide of the present invention has a titer of more than 1:8000, has high specificity to UL49 prokaryotic expression protein, eukaryotic expression protein and HCMV particles, and can be used to prepare in vitro diagnosis or treatment of human cytomegalovirus medicine. The antibody prepared by the antigen peptide of the present invention is the basic element of HCMV UL49 biological function research, and the HCMV infection can be detected qualitatively or quantitatively by using the antibody.
Owner:JINAN UNIVERSITY
Antibodies against human cytomegalovirus (HCMV)
The present invention provides novel antibody sequences that bind human cytomegalovirus (hCMV) and neutralize hCMV infection. The novel sequences can be used for the medical management of hCMV infections, in particular for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of hCMV infections.
Owner:RIBOVAX BIOTECHNOLOGIES SA
Identification of an altered therapeutic susceptibility to Anti-hcmv compounds and of a resistance against Anti-hcmv compounds
InactiveUS20140193802A1Improve accuracyImprove reliabilityOrganic active ingredientsMicrobiological testing/measurementHCMV InfectionPyridine
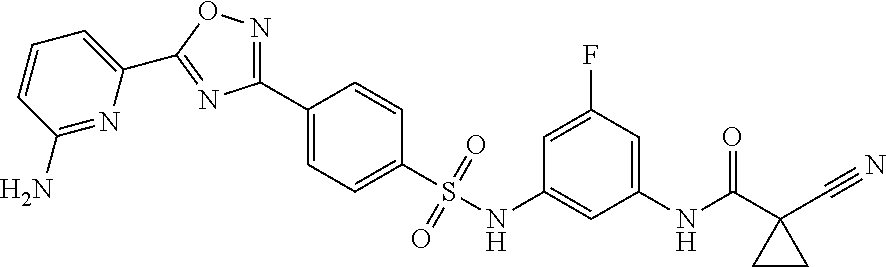
The present invention relates to a method for the detection of an altered therapeutic response of a subject infected by HCMV to a treatment with a 3,4 dihydroquinazoline or N-{3-[({4-[5-(6-aminopyridin-2-yl)-1,2,4-oxadiazol-3-yl]phenyl}sulfonyl)amino]-5-fluorophenyl}-1-cyanocyclopropanecarboxamide, a method for the detection of a drug resistance of a HCMV to a 3,4-dihydroquinazoline or N-{3-[({4-[5-(6-aminopyridin-2-yl)-1,2,4-oxadiazol-3-yl]phenyl}sulfonyl)amino]-5-fluorophenyl}-1-cyanocyclopropanecarboxamide, and to a method for the detection of a mutation of a HCMV resulting in a drug resistance to a 3,4-dihydroquinazoline or N-{3-[({4-[5-(6-aminopyridin-2-yl)-1,2,4-oxadiazol-3-yl]phenyl}sulfonyl)amino]-5-fluorophenyl}-1-cyanocyclopropanecarboxamide.
Owner:AICURIS GMBH & CO KG
Use of Ginseng Extract, Ginsenoside and Ginsenoside Derivative in the Preparation of Medicine or Health Care Product for Treating Cytomegalovirus Infection Disorders
The invention discloses new application of a ginseng extract, ginsenoside and a ginsenoside derivative in the preparation of a medicine for treating HCMV infection related diseases. The related diseases comprise HCMV infection caused in the process of treating cardiovascular and cerebrovascular diseases, organ transplantation, perinatal periods, tumors, burn, AIDS and other diseases of patients. Tests prove that the ginseng extract, the ginsenoside and the ginsenoside derivative have a remarkable curative effect, quick response and a small toxic or side effect during treatment of HCMV infected diseases, are a medicine or a health product for treating the HCMV infection, which is safe, efficient and stable and has a simple preparation process, and are suitable for industrial production and easy to popularize. In the invention, a new medicine source is provided for the treatment of the HCMV infection.
Owner:FU LI
Compositions and methods for treatment of cytomegalovirus
InactiveCN104271745ASsRNA viruses negative-senseAntibody mimetics/scaffoldsEpitopeVirus-like particle
The present disclosure provides compositions and methods useful for treating HCMV infection. As described herein, the compositions and methods are based on development of immunogenic compositions that include virus-like particles (VLPs) which comprise one or more Moloney Murine leukemia virus (MMLV) core proteins and include one or more HCMV epitopes, such as, for example, from HCMV envelope glycoproteins gB and / or gH and / or tegument protein pp65. Among other things, the present invention encompasses the recognition that a combination of antigens (e.g., envelope glycoproteins and structural proteins) can lead to beneficial immune responses, for example that include both a humoral response (e.g., production of neutralizing antibodies) and a cellular response (e.g., T-cell activation).
Owner:VARIATION BIOTECHNOLOGIES INC
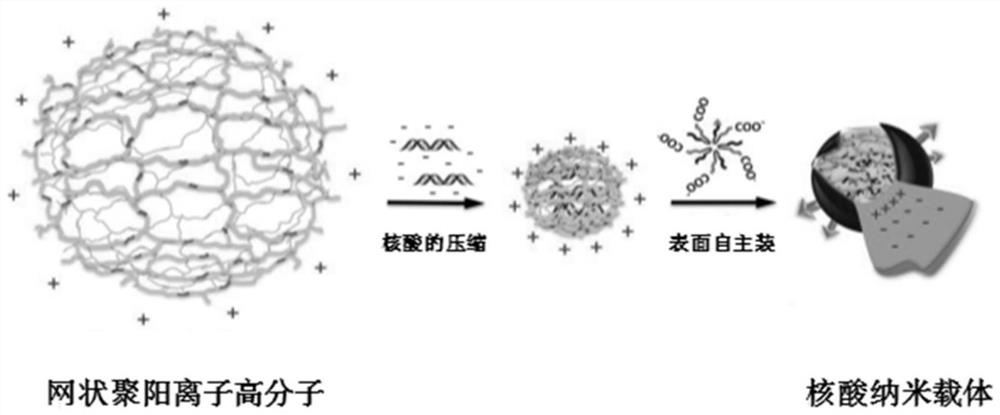
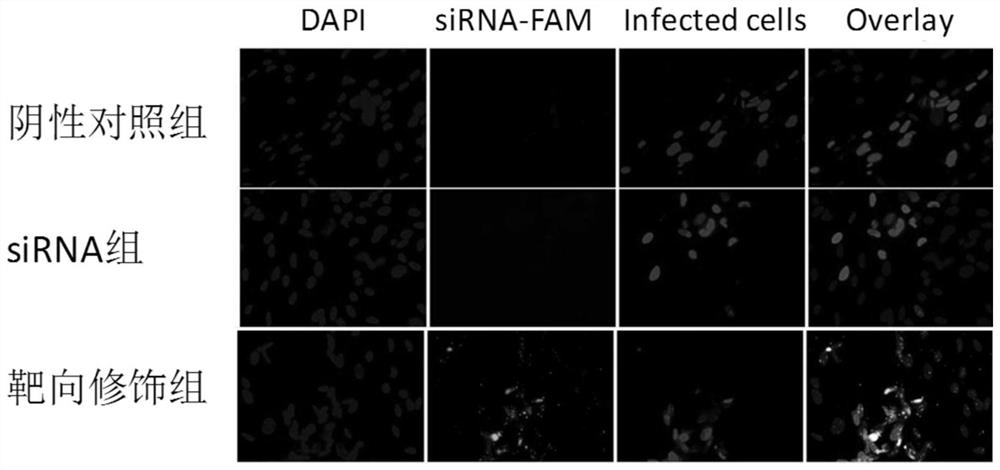
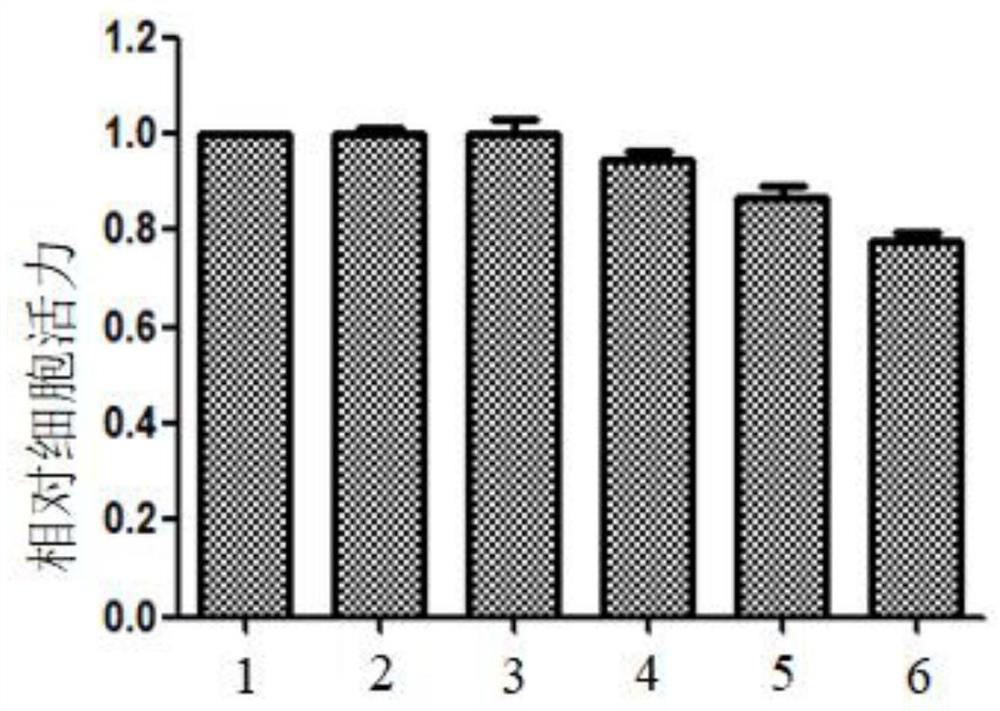
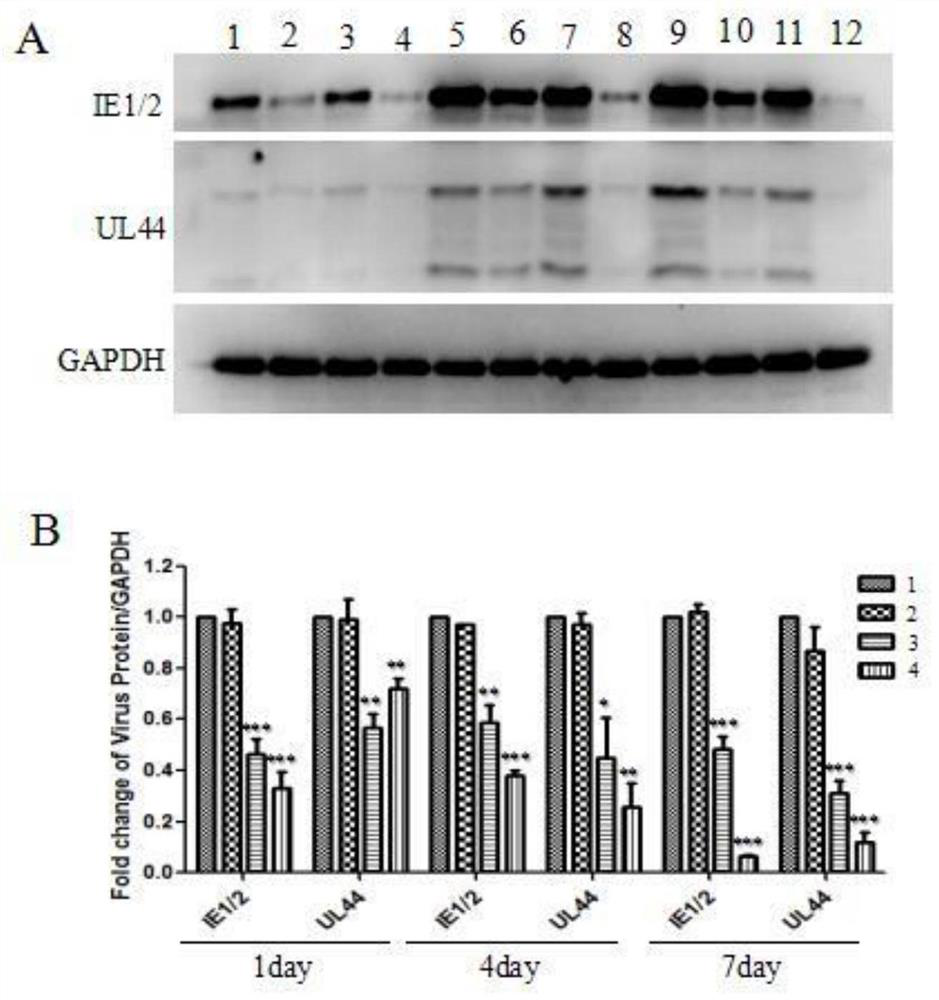
Small nucleic acid for inhibiting human cytomegalovirus infection as well as preparation and application thereof
PendingCN112175948AImprove phagocytosis efficiencyIncrease local concentrationPowder deliveryOrganic active ingredientsHCMV InfectionTherapeutic effect
The invention discloses small nucleic acid for inhibiting human cytomegalovirus infection as well as a preparation and application thereof. The small nucleic acid for inhibiting human cytomegalovirusinfection has nucleotide sequences as shown in SEQ ID NO.1-3. According to the invention, the small nucleic acid is mainly used as a therapeutic drug to prevent further expression of related proteinsof HCMV-related genes, and meanwhile, targeting molecules capable of being combined with the small nucleic acid are designed for surface characteristics of virus-infected cells to improve the phagocytic efficiency of an action site of the small nucleic acid, so that the treatment concentration of the lesion action site is improved, the treatment effect is enhanced, meanwhile the uptake rate of thenon-target tissue is reduced to reduce the potential toxicity, and an effective solution is provided for HCMV infection in-vivo treatment.
Owner:JINAN UNIVERSITY
New Application of Corydalin in Prevention and Treatment of Human Cytomegalovirus Infection
Owner:ZHEJIANG HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com