Small nucleic acid for inhibiting human cytomegalovirus infection as well as preparation and application thereof
A technology of human cytomegalovirus and small nucleic acid, which is applied in the field of small nucleic acid and its preparation and application to inhibit human cytomegalovirus infection, and can solve problems such as inability to respond quickly, lack of selective delivery of infected cells, and poor carrier stability , to achieve the effect of improving drug effect, improving therapeutic effect and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Screening of siRNA
[0062] For HCMV virus early protein (Immediate early protein) IE1 protein (HCMV IE1:>sp|P03169|VIE1_HCMVT 55kDa immediate-early protein 1OS=Human cytomegalovirus (strain Towne) OX=10363GN=UL123 PE=1SV=1; Link: https: / / www.uniprot.org / uniprot / P03169) (SEQ ID NO.5) and IE2 protein (HCMV IE2:>tr|Q6SWJ2|Q6SWJ2_HCMVORegulatory protein IE2 OS=Human cytomegalovirus (strain Toledo) OX=311339GN=UL122 PE=4SV =1; Link: https: / / www.uniprot.org / uniprot / Q6SWJ2) (SEQ ID NO.6) mRNA expressed, a small nucleic acid is designed, which can bind to mRNA with high specificity and block the expression of related proteins Expression, and then achieve the therapeutic effect of inhibiting virus replication; at the same time, in order to reduce the off-target effect, according to the existing literature reports (reference 1: Hamilton S T, Jens M, Manfred M, et al. Human Cytomegalovirus Replication Is Strictly Inhibited by siRNAs Targeting UL54, UL97 or UL122 / 123 G...
Embodiment 2
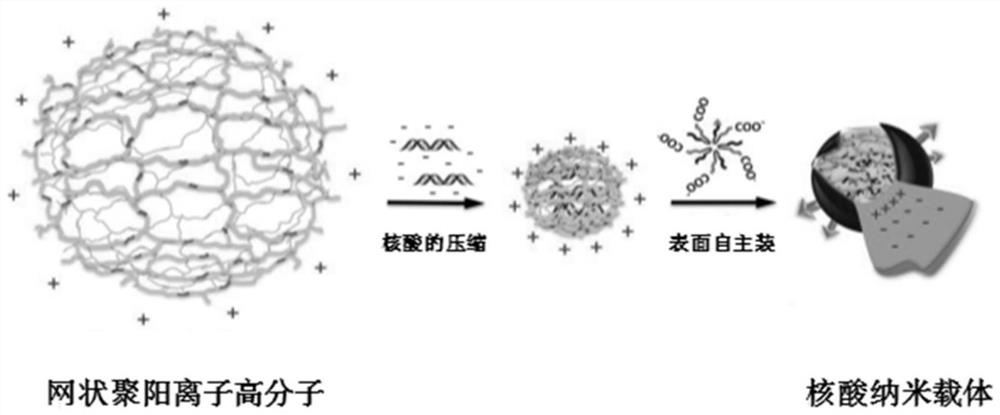
[0070] Example 2 Synthesis of block polymers and preparation of nanoparticles
[0071] Mix the scRNA and siRNA (siRNA-1, siRNA-2) in Example 1 at a molar ratio of 1:1:1, and then prepare nanoparticles. The prepared nanoparticles have a "nuclear-membrane" structure and can be independently Packed as a nanoscale nucleic acid carrier; wherein, the core material synthesis involved, the surface material synthesis method, and the nanoparticle preparation method are as described in the references (Feng J, ChenS, Ge X, et al.Precise Assembly of Synthetic Carriers of siRNA through a Series of Interlocked Thermodynamically Self-Regulated Processes[J].Advanced Functional Materials,2017,28(6):1703207.).
[0072] The core material is networked cationic polymer, and the surface material is polyethylene glycol-polycaprolactone-carboxy-modified maltotriose block polymer PEG-PCL-Maltotriose-COOH (including five Fluorophenol-modified polyethylene glycol block polymer PFP-PEG-PCL-Maltotriose-CO...
Embodiment 3
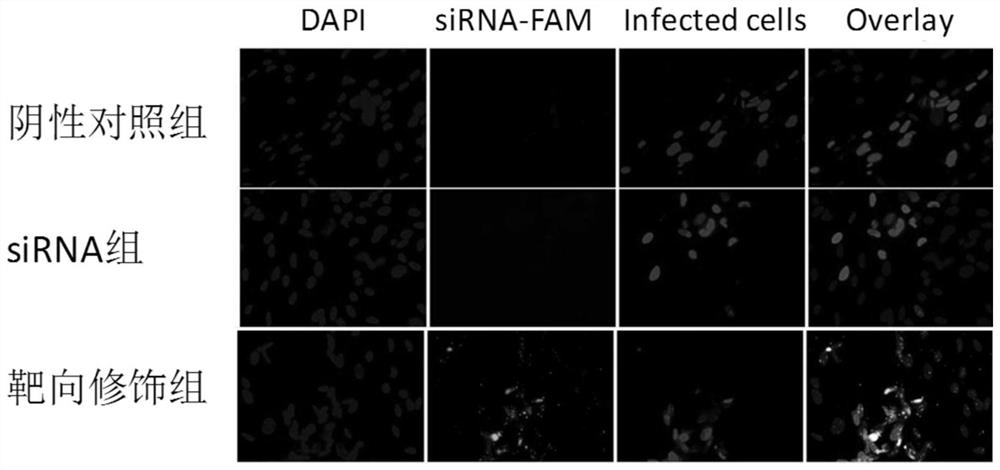
[0077] The effect of embodiment 3 targeting molecules
[0078] (1) Link targeting group
[0079] Through the formulation method, we link the targeting molecule CX that can specifically recognize HCMV-infected cells through chemical covalent bonds 3 CL 1 , the polypeptide sequence was obtained by solid-phase synthesis, and its sequence was: QHHGV-TKCNITCSKMTSKIPVALLIHYQQNQAS-CGKRAIILETRQHRLACADPKEQWVKDAMQHLDRQAAALTRNG (SEQ ID NO.4), and Fmoc protection was carried out to the biologically active end (the polypeptide was synthesized by Shanghai Jier Polypeptide Company), and lysine was connected to one end of the polypeptide The amino group of the acid is chemically linked with the pentafluorophenol in the polymer material forming nanoparticles, and the specific steps are:
[0080] ① Get 15mg block polymer PFP-PEG45-PCL-Maltotriose-COOH (i.e. the polyethylene glycol-polycaprolactone-carboxyl modified maltotriose block polymer in Example 2) (2.5mmoL), then add Equimolar CX 3 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com