Tumor-specific cleavable pegized nanoparticles, preparation method and application
A tumor-specific, nanoparticle technology, applied in the field of tumor-specific cleavable PEGylated nanoparticles and their preparation, can solve problems such as prolonging circulation time, reducing drug targeted release and bioavailability, and achieving biocompatibility Good properties, strong drug loading capacity, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
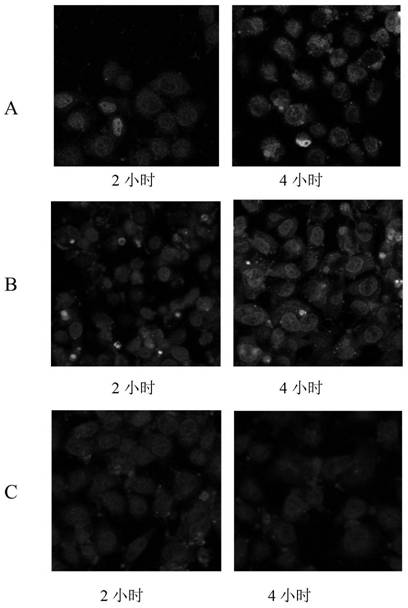
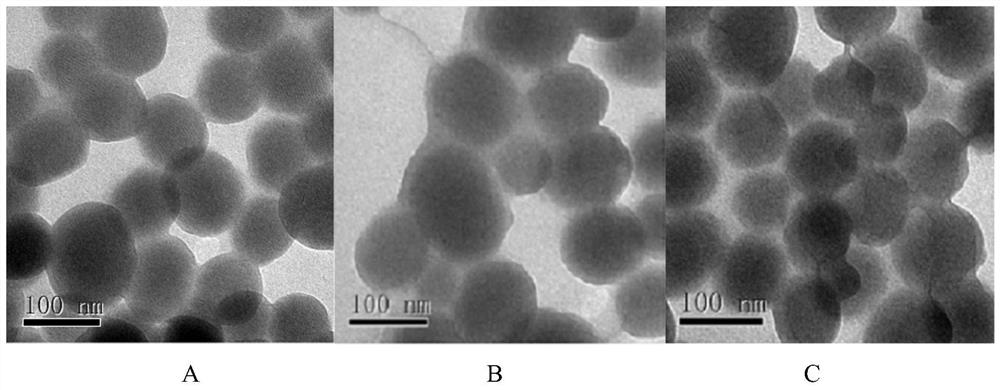
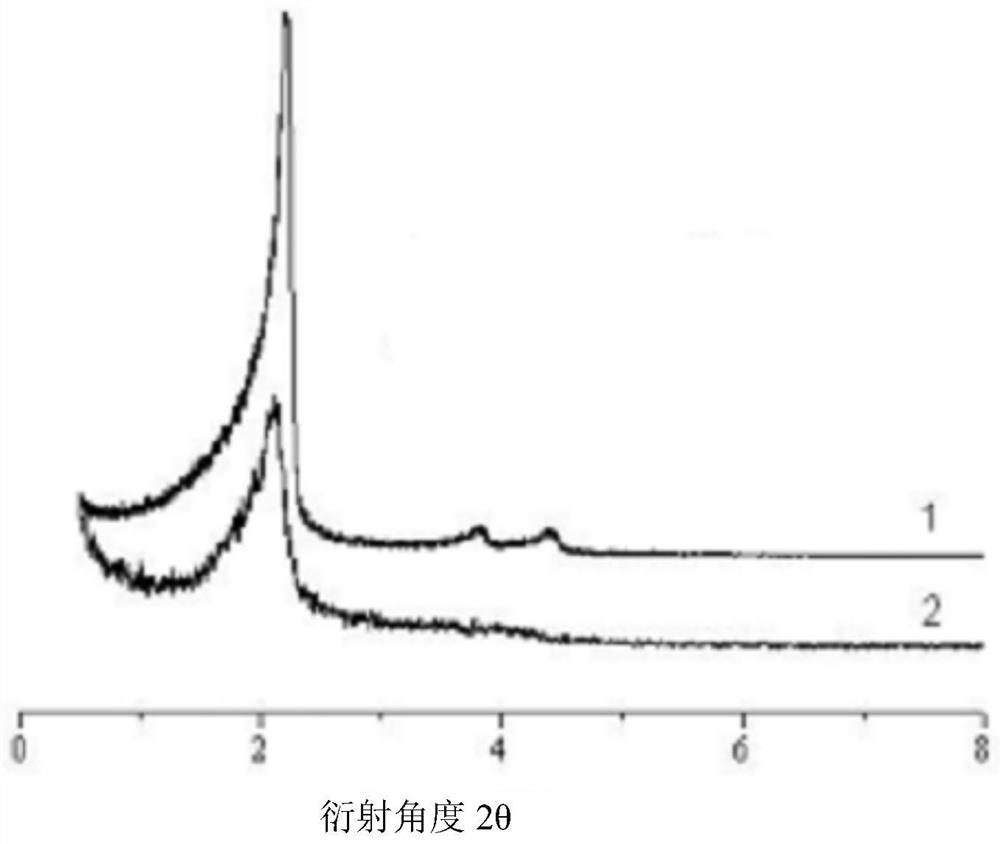
[0072] (1) Pre-synthesized MCM-41 type mesoporous silicon nanoparticles (synthesized according to the method in Colloids and Surfaces B: Biointerfaces, 2017, 155:41–50., with a particle size of 100nm and a pore size of 2-10nm) 60 After vacuum drying at ℃ for 24 hours, weigh 0.50 g and add it to 50 mL of anhydrous toluene, then add 0.20 mL of γ-aminopropyltriethoxysilane, stir evenly, raise the temperature to 110 ℃, and reflux for 24 hours under the protection of nitrogen. After the reaction was completed, it was filtered with suction, washed twice with toluene and once with ethanol, and dried in vacuum at 60° C. for 24 hours to obtain amino group-containing mesoporous silicon nanoparticles. Disperse 0.10 g of amino group-containing mesoporous silicon nanoparticles in acetone, add 0.20 g of succinic anhydride, stir and react at room temperature for 24 hours, and centrifuge to obtain carboxyl group-containing mesoporous silicon nanoparticles. Disperse 0.10g of carboxyl-containin...
Embodiment 2
[0078]The difference between embodiment 2 and embodiment 1 mainly lies in: this implementation step (4) and (5) replace the aminopolyethylene glycol monomethyl succinate of embodiment 1 with monomethoxy polyethylene glycol succinimide succinate Ether, so that the surface-coupled PEG can be broken into a stable covalent bond under slightly acidic conditions.
[0079] (1) Pre-synthesized MCM-41 type mesoporous silicon nanoparticles (Colloids and Surfaces B: Biointerfaces, 2017, 155:41–50.) (particle size 100nm, pore size 2-10nm) were vacuum-dried at 60°C for 24h , weighed 0.50g and added to 50mL of anhydrous toluene, then added 0.20mL of γ-aminopropyltriethoxysilane, stirred evenly, raised the temperature to 110°C, and refluxed for 24h under nitrogen protection. After the reaction was completed, it was filtered with suction, washed twice with toluene and once with ethanol, and dried in vacuum at 60° C. for 24 hours to obtain amino group-containing mesoporous silicon nanoparticle...
Embodiment 3
[0084] The difference between Example 3 and Example 1 mainly lies in that in this implementation step (3), N,N-diethylacrylamide is substituted for the N,N-dimethylacrylamide in Example 1.
[0085] (1) Vacuum-dry the pre-synthesized MCM-41 type mesoporous silicon nanoparticles at 60°C for 24 hours, weigh 0.50 g and add it to 50 mL of anhydrous toluene, then add 0.20 mL of γ-aminopropyltriethoxysilane, and stir evenly. The temperature was raised to 110° C., and the reaction was refluxed for 24 hours under the protection of nitrogen. After the reaction was completed, it was filtered with suction, washed twice with toluene and once with ethanol, and dried in vacuum at 50° C. for 24 hours to obtain amino group-containing mesoporous silicon nanoparticles. Disperse 0.10 g of amino group-containing mesoporous silicon nanoparticles in acetone, add 0.20 g of succinic anhydride, stir and react at room temperature for 24 hours, and centrifuge to obtain carboxyl group-containing mesoporou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com