An MVA vaccine for delivery of a UL128 complex and preventing CMV infection
A complex and vaccine technology, applied in the field of vaccine compositions for preventing HCMV infection, preventing HCMV from invading cells, and treating HCMV infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: A Vaccine Introducing Broadly Neutralizing Antibodies in CMV Nascent Rhesus Monkeys that Inhibits the Main Entry Point of CMV Infection in Rhesus Monkeys
[0077] Viruses and cells. MVA propagation in baby hamster kidney (BHK) cells, and preparation and storage of virus stocks were performed according to previously reported protocols (Wang et al. 2010). Chicken embryonic fibroblasts (CEF) used for MVA propagation were maintained in serum-free medium for virus production (VP-SFM; Invitrogen).
[0078]MVA expressing a full-length pentamer of the five subunits RhUL128, RhUL130, RhUL131, RhgL, and RhgH (MVA-RhUL128C), with an alternate transmembrane (TM) site deletion, was generated by BAC technology as described below Versions of gH express the MVA of this 5-subunit pentamer (MVA-RhUL128C[Delta]), or the MVA of the RhUL128, RhUL130, and UL131A subunits. MVAs expressing RhUL128 or RhUL130 alone were generated by a routine protocol in eukaryotic cells as previou...
Embodiment 2
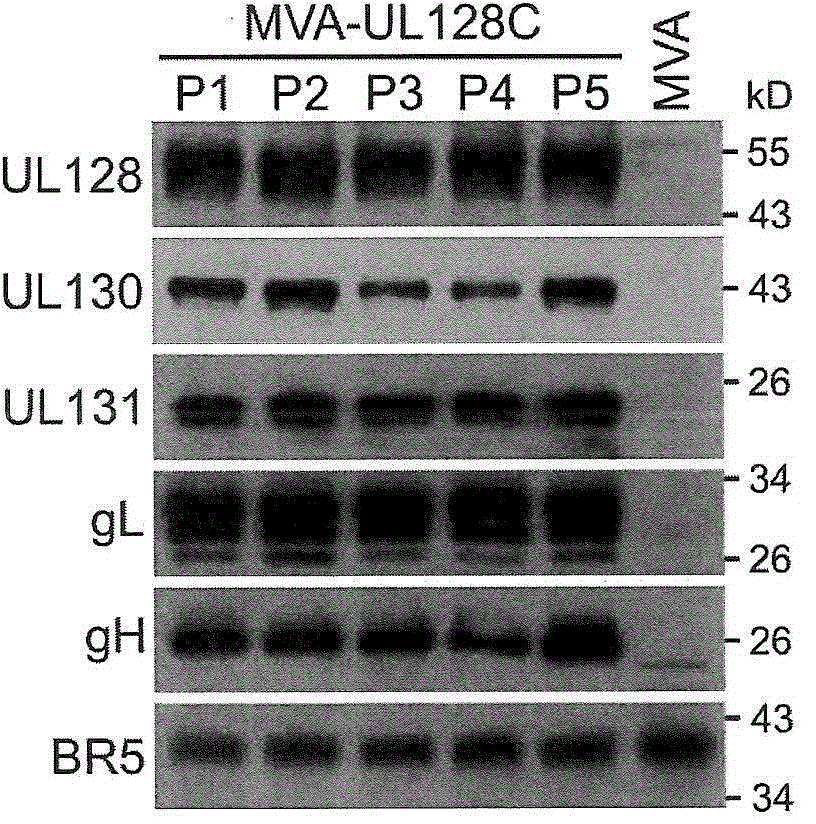
[0118] Example 2: Construction and expression of HCMV UL128C pentamer expressed from MVA
[0119] MVA expression of the human UL128C subunit. MVA expressing 5 subunit pentamers of full length human UL128, UL130, UL131, gL and gH (H-UL128C-MVA) was generated by BAC technique similar to Example 1.
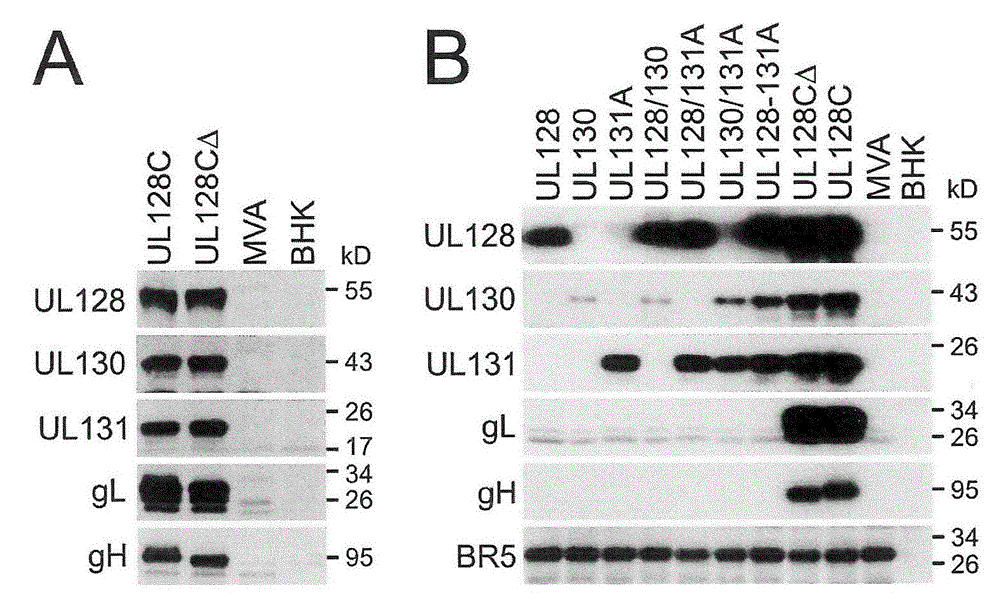
[0120] Such as Figure 8 As shown, all five subunits of human UL128C (H-UL128C) were successfully expressed and individual subunits were detected by either mAb (gH, UL128, UL130) or polyclonal antisera (UL131A, gL). The MWs of the individual subunits are consistent with published data, providing strong evidence that MVA-based expression is accurate and reliable for the structure of these subunits expressed from HCMV. Figure 8 Compositions containing alternating gH structures (e.g., gH, gHΔTM lacking the transmembrane region) co-expressed with the other four H-UL128C subunits are shown (a composition with alternating gHΔTM is represented by UL128CΔ, and Full-length gH (gH-FL) is...
Embodiment 3
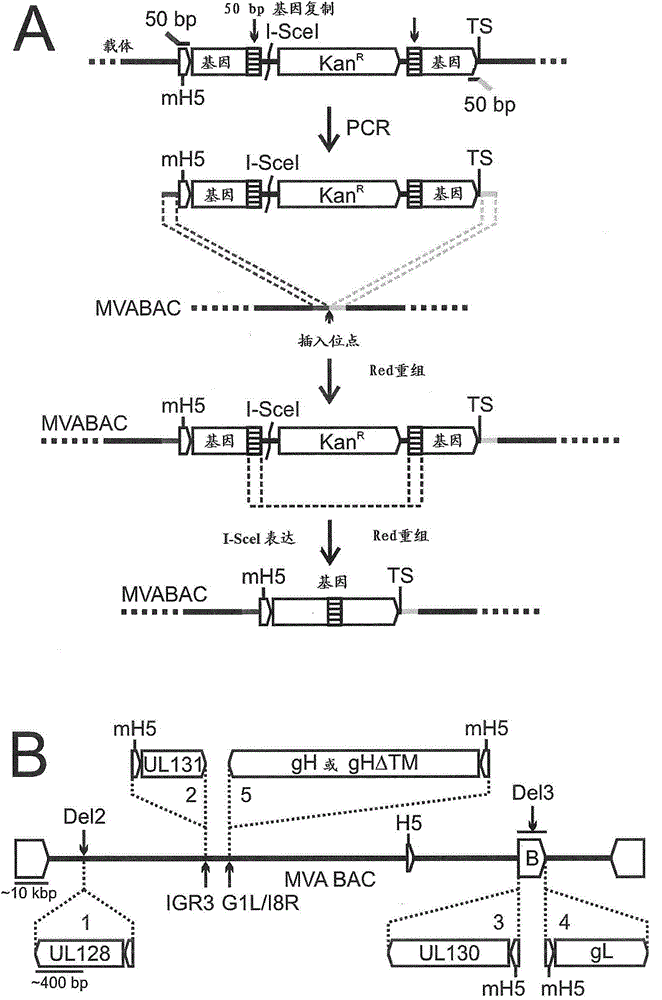
[0122] Example 3: Construction and evaluation of clinically acceptable MVA-BAC for human use
[0123] Preclinical studies with RhCMV were facilitated by a previously constructed MVA-BAC vector (Cottingham 2008). All vaccines using RhCMV compounds use this MVA-BAC combination. However, because the origin of MVA is unknown, a different vaccine vector will be used for human use. The MVA MBA will also be constructed in such a way that vector sequences can be deleted after viral recombination to avoid retention of any unwanted functional bacterial sequences, a property that may be necessary for FDA approval. The vector components will be inserted into the TK site to recombine the insertion site for transgene expression. The construction of MVA-BAC for humans was followed by using the 1974-MVA provided by Dr. Bernard Moss of NIAID. The clinical use of MVA has allowed the development of a variety of vaccines, many of which are now entering clinical trials. Therefore, 1974-MVA s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com