Preparation method and application of anti-HCMV (human cytomegalovirus) Pp65 protein monoclonal antibody
A monoclonal antibody and antigen technology, applied in the field of genetic engineering, can solve problems such as high price of diagnostic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Animal immunization and serum titer detection: Pp65 protein plantar plate immunization mice
[0037] The purified Pp65 protein was fully emulsified with complete Freund's adjuvant, and three BALB / c female mice aged 6-8 weeks were immunized with a dose of 100 μg each by plantar plate immunization method. Two weeks later, Pp65 recombinant protein was fully emulsified with Freund's incomplete adjuvant, and BALB / c mice were boosted with the same dose and method. The mouse serum was collected 1 day before each immunization, and the antibody titer in the serum was detected by indirect ELISA. Three days before the fusion, the plantar plates were immunized with Pp65 recombinant protein without adjuvant at a dose of 100 μg per mouse. The collected blood was diluted 1:5 in normal saline, and then the blood suspension was placed at 37°C for 2 hours, then moved to 4°C overnight, centrifuged at 4000 g for 10 minutes after hemagglutination, and the supernatant was absor...
Embodiment 2
[0041] Example 2: Cell Fusion, Positive Hybridoma Screening and Subcloning
[0042] The SP2 / 0 myeloma cells in good growth state and lymph node cells of immunized mice were selected, washed three times with serum-free medium, mixed at 1:5~1:10, centrifuged at 1500 g for 5 min, and the supernatant was discarded. After gently shaking the centrifuge tube to disperse the cells evenly, slowly add 1 mL of 37 ℃ preheated PEG 1 500 and mix gently, then add 20 mL of 1640 medium. After centrifugation at 800 g for 5 min, discard the supernatant, add 20% FBS-HAT-1640 medium to suspend the cells and mix well.
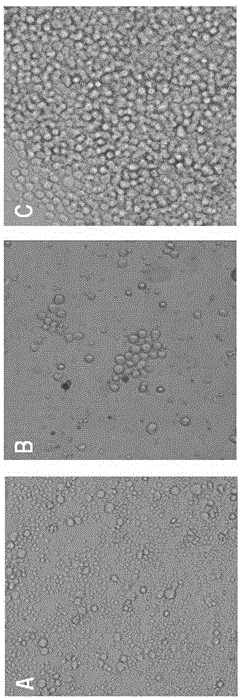
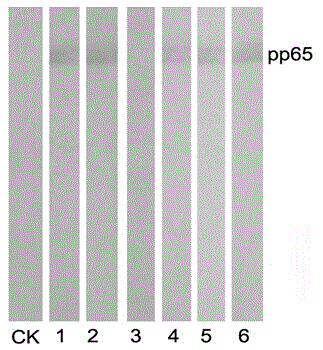
[0043] The mixed cells were spread evenly in 96-well plates and placed at 37 °C, 5% CO 2 Cultured in an incubator, follow up and observe every day, change the medium with 10% FBS-HT-1640 culture medium 7-10 days after fusion, and detect with indirect ELISA when the clone grows to 1 / 3-1 / 2 of the bottom area of the well For the antibody in the supernatant of hybridoma cells, th...
Embodiment 3
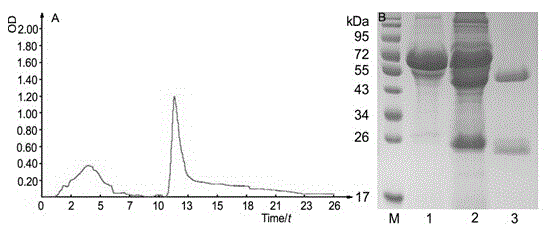
[0047] Example 3: Production and purification of monoclonal antibodies
[0048]The monoclonal antibody was produced by inducing ascites in mice. Take 8-10 w BALB / c mice, and inject sterile paraffin oil into the intraperitoneal cavity, 0.5 mL each. After 3 days, the 8D6 hybridoma cell line was injected into the abdominal cavity, and the cell density was adjusted to 1×10 6 ~2×10 6 / mL, each mouse was intraperitoneally injected with 0.5 mL of cell suspension. Seven to 12 days after cell inoculation, abdominal swelling of the mice could be clearly seen, and the ascites was collected and purified. The collected ascites was firstly purified by adding saturated ammonium sulfate precipitation at 1:1, centrifuged at 12,000 g for 10 min at 4°C, discarded the supernatant, and used a certain volume of PBS buffer (20 mmol / L NaCl, 2.68 mmol / L KCl, 10 mmol / L Na 2 HPO 4 , 1.76 mmol / L KH 2 PO 4 , pH 7.4) were dissolved, dialyzed twice into 10 times the volume of PBS buffer, 2 h each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com