Antibodies against human cytomegalovirus (hcmv)
一种抗体、抗体片段的技术,应用在抗病毒剂、抗病毒免疫球蛋白、抗体等方向,能够解决没有发现临床有效性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
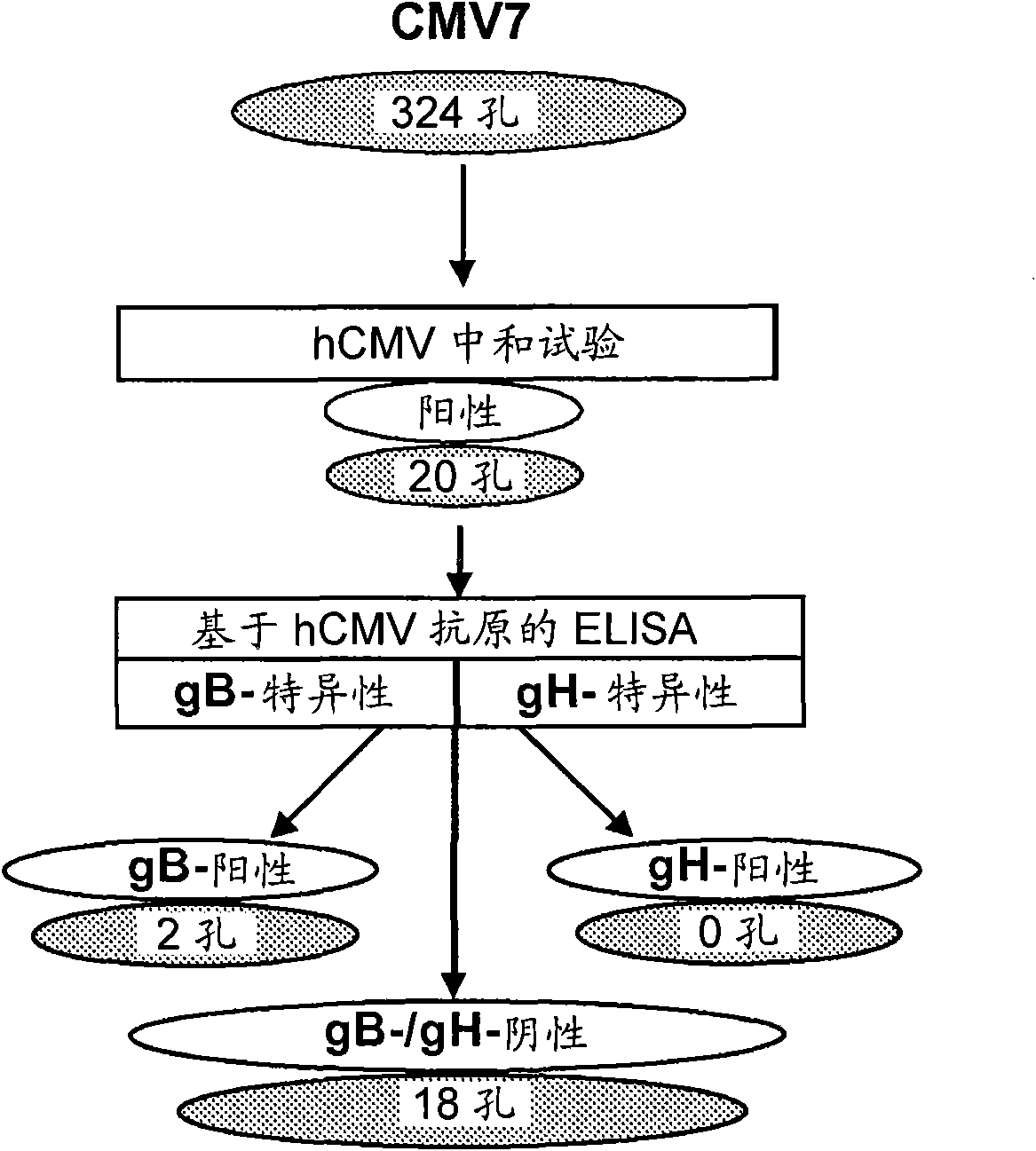
[0092] Example 1: Culture of cells secreting human monoclonal antibodies that neutralize hCMV infectivity production of things
[0093] Materials and Methods
[0094] Selection of human donors with hCMV-neutralizing IgG antibodies in serum
[0095]hCMV-specific detection was performed as outlined in PCT / EP2005 / 056871 and in the literature summarized below.
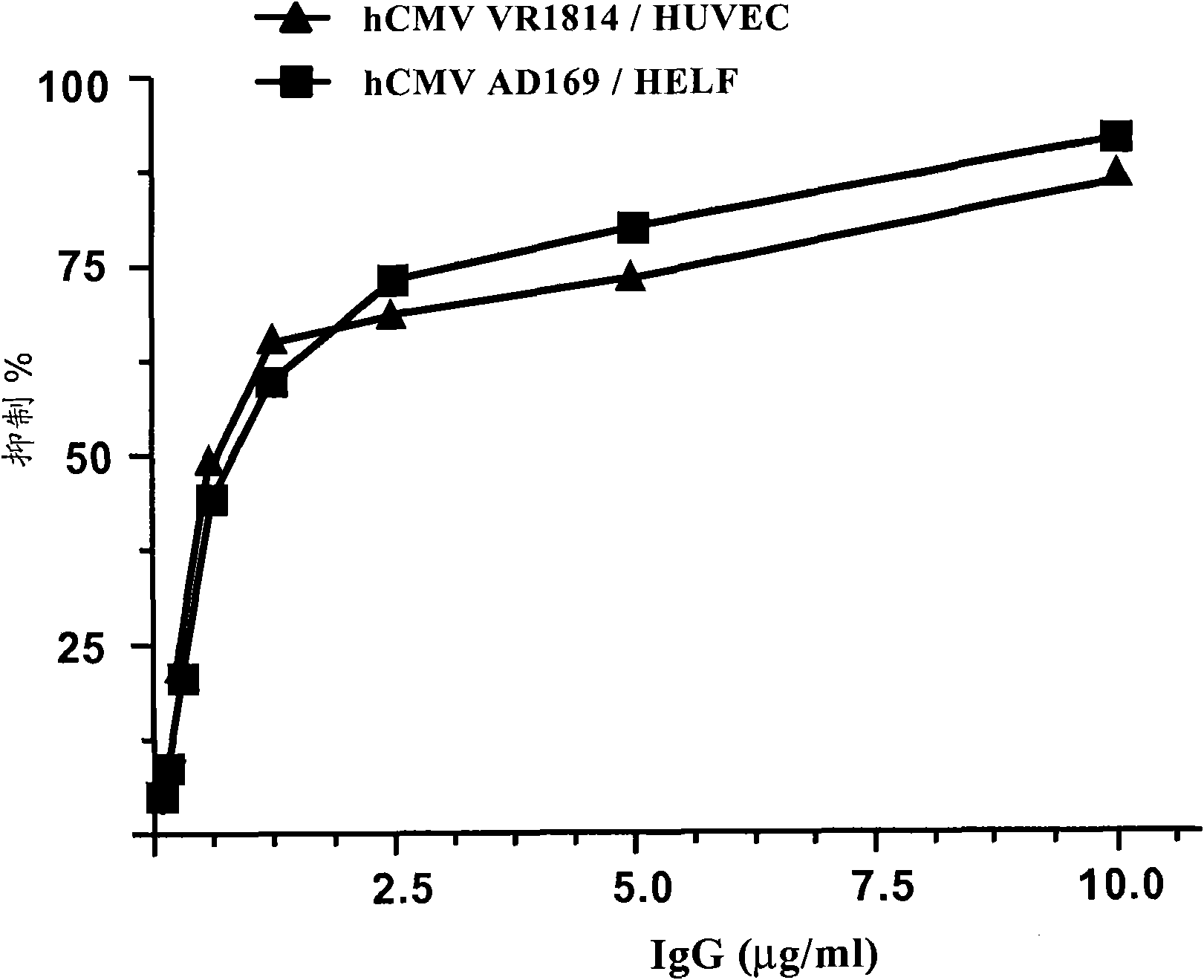
[0096] hCMV neutralizing antibodies were detected according to the hCMV microneutralization assay based on human embryonic lung fibroblasts (HELF cells) and hCMV AD 169 strain (ATCC laboratory strain of hCMV, number VR-538).
[0097] It can also be performed using the endotheliotropic hCMV VR1814 strain (Revello M et al., 2001) and human umbilical vein endothelial cells (HUVEC) as progeny of clinical isolates recovered from cervical swabs of pregnant women. hCMV microneutralization test. Enzyme treatment of the umbilical cord vein and growth in the presence of 2% fetal bovine serum (FBS), recombinant human vascula...
Embodiment 2
[0121] Example 2: Identification of 26A1 Antibody
[0122] Materials and methods
[0123] Expansion and identification of 26A1 subculture
[0124] Cells from the original subculture 26A1 were expanded on radioactive allogeneic PBMCs in IMDM medium (supplemented with 10% FCS and NEAA), using during this expansion step as described in Example 1 The hCMV microneutralization assay confirmed hCMV neutralizing activity at least twice (see Table 1).
[0125] The amount of antibody secreted by 26A1 subcultures at 24, 48 and 72 hours was determined using a commercially available quantitative human IgG ELISA kit (Immunotek; Cat. No. 0801182; Zeptometrix Corp.) according to the manufacturer's instructions. The subclass of the 26A1 antibody was determined using a commercially available assay (PeliClass human IgG subclass ELISA combi-kit; number #RDI-M155lcib, RDI Division of Fitzgerald Industries Intl.).
[0126] Cells contained in 1 well of a 96-well plate (≈1 × 10 5 ) were inocu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com