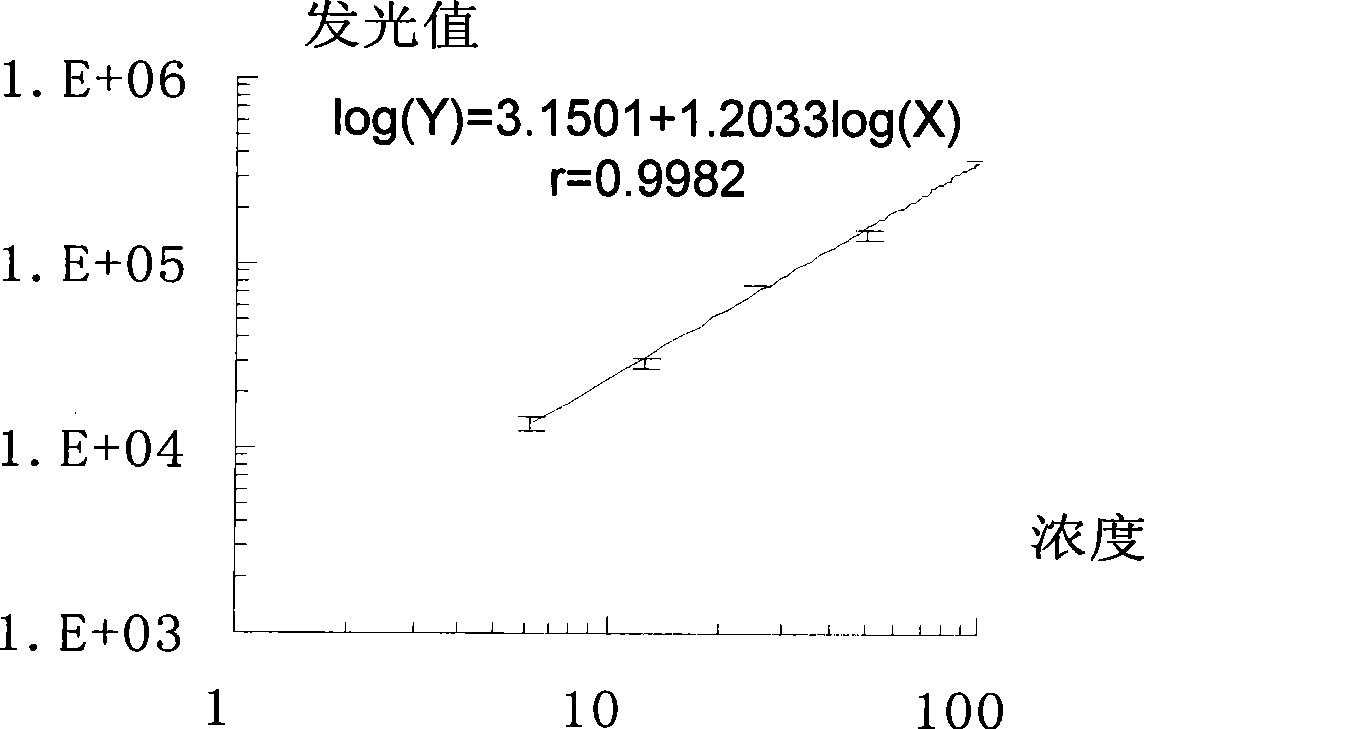
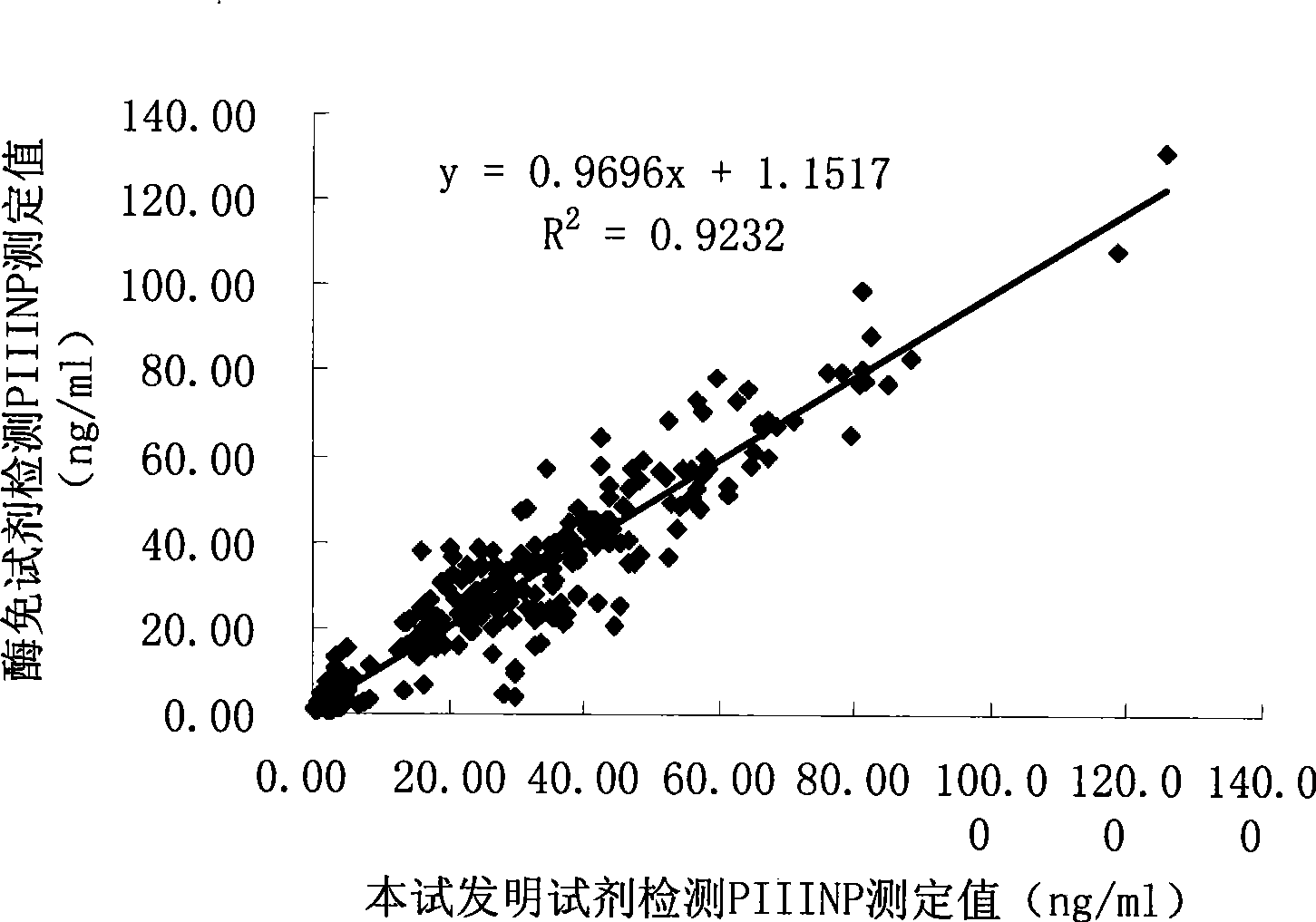
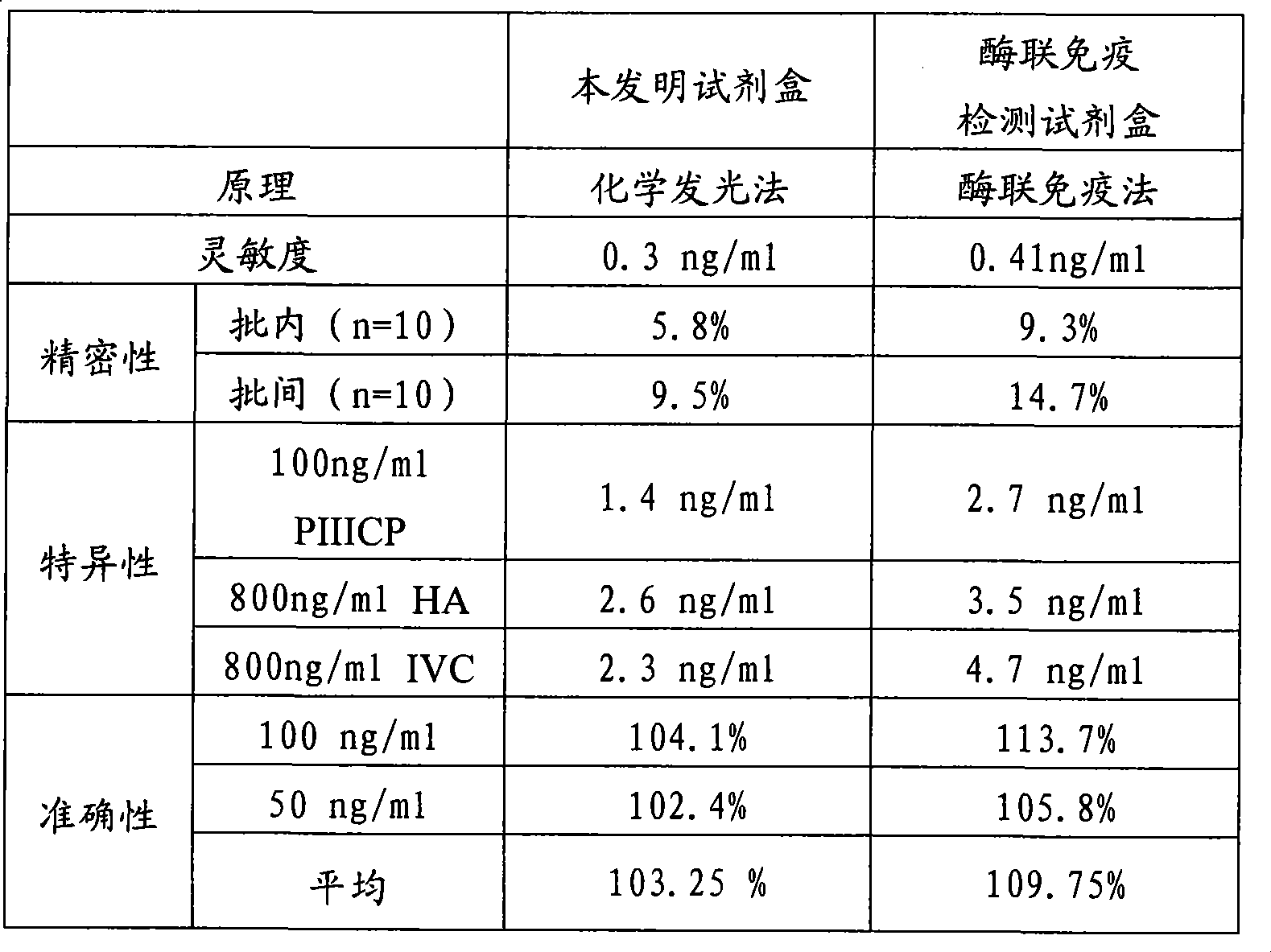
III type precollagen N end peptide chemiluminescence immune analysis quantitative determination reagent kit and preparing method thereof
A chemiluminescence immunity and chemiluminescence technology, which is applied in the field of immunoanalysis medicine, can solve the problems of inability to be widely used in clinical diagnosis and scientific research work, needs, and restrictions on promotion and use, so as to ensure sensitivity, increase sensitivity, and facilitate operation and production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of the type III procollagen N-terminal peptide quantitative assay kit of the present invention
[0030] 1. Preparation of enzyme-labeled antibody
[0031] Type III procollagen N-terminal peptide monoclonal antibody was coupled with alkaline phosphatase by the glutaraldehyde method, fully dialyzed against PBS, added an equal volume of glycerol, and stored below -20°C.
[0032] 2. Preparation of type III procollagen N-terminal peptide calibrator
[0033] Prepared with pure N-terminal peptide of type III procollagen, divided into 6 bottles of 0, 6.2, 12.5, 25, 50, 100ng / ml.
[0034] 3. Enzyme-labeled antibody concentration selection
[0035] Use the square array method to select the working concentration of the enzyme-labeled antibody to be greater than 1:6000.
[0036] 4. Preparation of solid-phase coated plates
[0037] (1) Coated
[0038] Trisodium citrate 2.92g
[0039] Citric acid 1.78g
[0040] Double distilled water 400ml
[0041] After...
Embodiment 2~3
[0071] Examples 2-3 Preparation of the Type III procollagen N-terminal peptide quantitative assay kit of the present invention
[0072] Divided by horseradish peroxidase-labeled type III procollagen N-terminal peptide monoclonal antibody, with luminol (luminol) system as chemiluminescent substrate, this chemiluminescent substrate includes A liquid and B liquid, based on 1000mL of the chemical Luminescence substrate A solution, including 1.7716g luminol, 0.051g 4-hydroxybiphenyl, 0.012g 4-iodophenylboronic acid, 11.4g boric acid, 4.9g borax, its pH value is 8.0-10.0; based on 1000mL of the chemical Luminescent substrate solution B, including 0.329g carbamide peroxide, 1ml Tween20, 51.58g Na 2 HPO 4 12H 2 O, 8.74gNaH 2 PO 4 2H 2 O, its pH value is 7.0 ~ 7.6, with plastic beads and plastic tubes as solid phase carriers respectively, except that the pH value of the coating solution is 4.5, and the pH value of the blocking solution is 7.5, the rest are all in the same way as i...
Embodiment 4
[0073] Example 4 Preparation of the type III procollagen N-terminal peptide quantitative assay kit of the present invention
[0074] Except that the magnetic particles were used as the carrier, and the magnetic particles were coated by the glutaraldehyde method, the rest were prepared in the same way as in Example 1 to prepare the type III procollagen N-terminal peptide quantitative assay kit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com