Human occludin antigen epitope peptide, antigen, antibody, kit and use
A protein antigen, epitope peptide technology, applied to the antibody in the in vitro diagnostic kit for human atresia, sex antigen and corresponding monoclonal antibody or polyclonal antibody, the field of human atresia, can solve the BBB Barrier function damage and other problems, to achieve the effect of assessing the degree of damage and reducing serious consequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
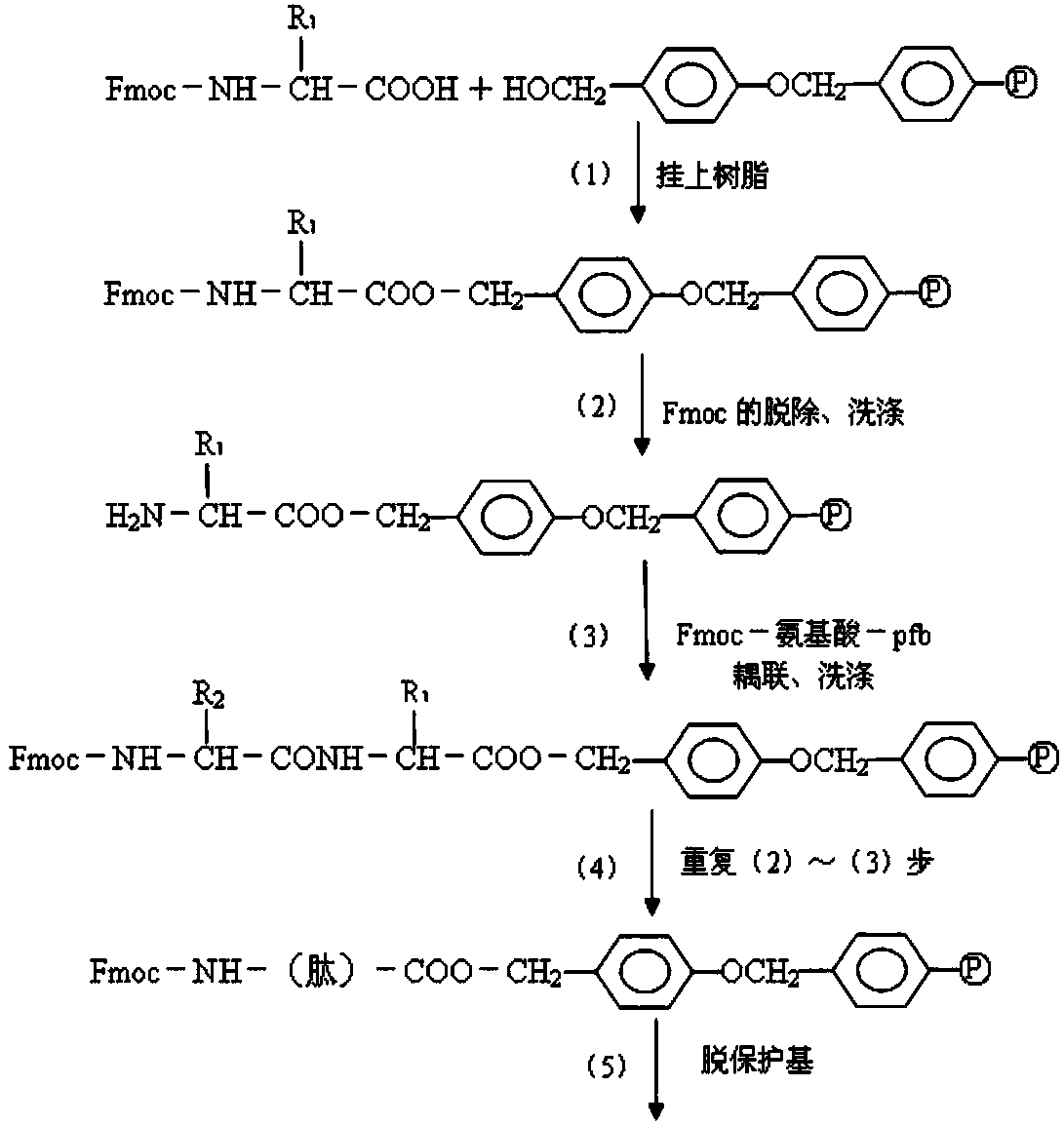
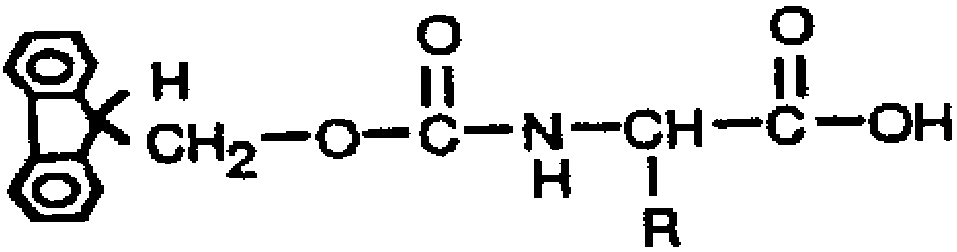
[0037] The preparation method of the occludin antigenic epitope peptide of the present invention can be a chemical synthesis method: the antigenic epitope peptide is synthesized by a solid-phase method using an American ABI431A type polypeptide automatic synthesizer. The molecular weights of the antigenic epitope peptides (1) and (2) of the present invention are 1971.13 and 2124.44 respectively, which can be determined by mass spectrometry, and the synthesized antigenic epitope peptide sequences are identified by polypeptide sequence determination. The purity of the peptides was evaluated by thin-layer chromatography and high-performance liquid chromatography, and the concentration of the epitope peptide was determined.
[0038] 2. Occlusin antigen
[0039] The present invention also provides an atretin antigen prepared by coupling one of the human atretin epitope peptides (1) and (2) of the present invention with a carrier protein. Specifically, the present invention provide...
Embodiment 1
[0054] Example 1: Preparation of Octrein epitope peptides (1) and (2).
[0055] The preparation method uses chemical synthesis method: using the American ABI431A automatic peptide synthesizer, the occludin epitope peptides (1) and (2) are synthesized respectively by solid-phase method. The purity of the epitope peptide was assessed by high performance liquid chromatography, and the concentration of the peptide was determined. The molecular weights of the antigenic epitope peptides (1) and (2) of the present invention are 1971.13 and 2124.44 respectively, which are determined by mass spectrometry, and the synthesized polypeptide sequences are identified by polypeptide sequence determination.
[0056] 1. Synthesis of Occlusin Epitope Peptides (1) and (2)
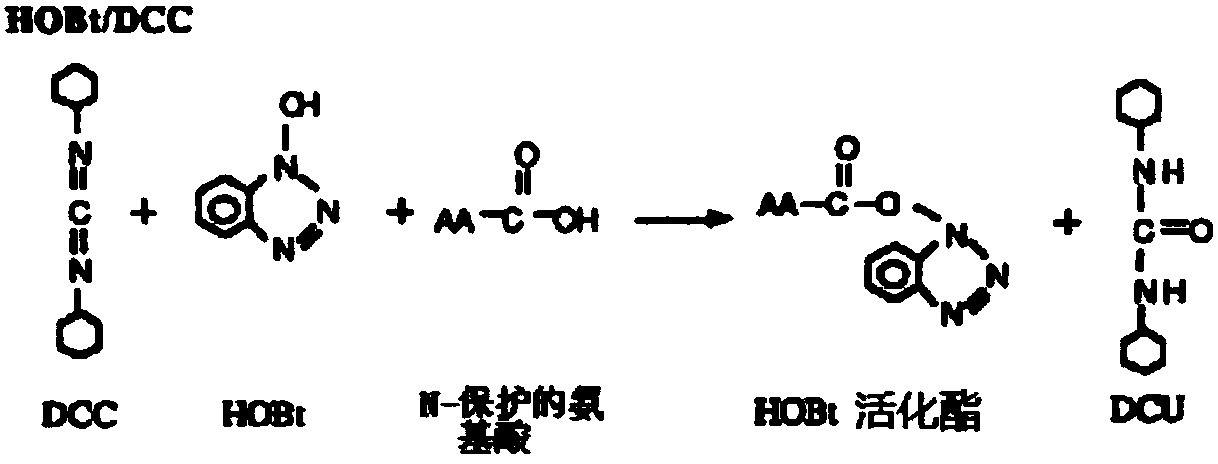
[0057] The above peptides were synthesized by solid-phase method. The main idea of solid-phase peptide synthesis is: first connect the carboxyl group of the carboxyl-terminal amino acid of the peptide chain to be synthesiz...
Embodiment 2
[0145] Example 2: The atretin antigen epitope peptides (1) and (2) obtained in Example 1 were linked to carrier proteins to prepare atretin antigens (1) and (2), and the obtained antigens (1) and (2) were used ) to immunize animals respectively, thereby using the antigen (1) to prepare specific monoclonal antibodies and polyclonal antibodies, and using the antigen (2) to prepare specific monoclonal antibodies and polyclonal antibodies.
[0146] 1. Antigen preparation: Occlusin peptides (1) and (2) were respectively linked with carrier protein KLH (keyhole limpet hemocyanin) (obtained from sigma company) by BDB (Bis-diazotizedbenzidine dichloride) method to prepare atocrin Antigens (1) and (2).
[0147] Take 10.0mg of Occlucin peptide (1) or (2), dissolve it with 1ml 0.1M PBS buffer (pH 7.4); dissolve 10mg of KLH with 20ml of 0.2M borate buffer (pH 9.0); then mix the two , cooled to 0°C, take BDBCl 2 110 μL, reacted at room temperature for 1.5 h, dialyzed overnight, then ali...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com