Primer for detecting RT-PCR of Seneca Valley viruses and RT-PCR detecting method
A technology of RT-PCR and detection method, which is applied in the field of RT-PCR detection primers of Seneca Valley virus, which can solve the problems of not being able to take timely and effective prevention and control measures, not having fast and effective detection methods, and not being able to diagnose pathogens in a timely manner , to achieve good application value, fast detection speed and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Primer design: Since Seneca Valley virus is a newly discovered pathogenic virus, common PCR detection technology has not been established in China at that time, and the gene sequences that can be found on GenBank are also extremely limited. We will use the only Seneca virus on GenBank The gene sequences of Valley virus SVV001 strain and 11-55910-3 strain were compared, and compared with the gene sequences of various viruses of the same virus family, and the conserved region was found, and the 1336-1662 position of SVV001 strain was selected Gene fragments are used as targets, and then BLAST further confirms their conservation. A pair of primers SVVUP / SVVDN with a 327bp amplification target band were designed on the conserved sequence through the Oligo6.0 primer design software. The primer sequences are as follows:
[0034] SVVUP: 5'-GATGTATAAACCTTCTC-3' (SEQ ID NO: 1)
[0035] SVVDN: 5'-ATTGTAAGTGCCAAGAG-3' (SEQ ID NO: 2).
[0036] 2. Extraction of sample RNA: Use ...
Embodiment 2
[0041] Embodiment two specificity test
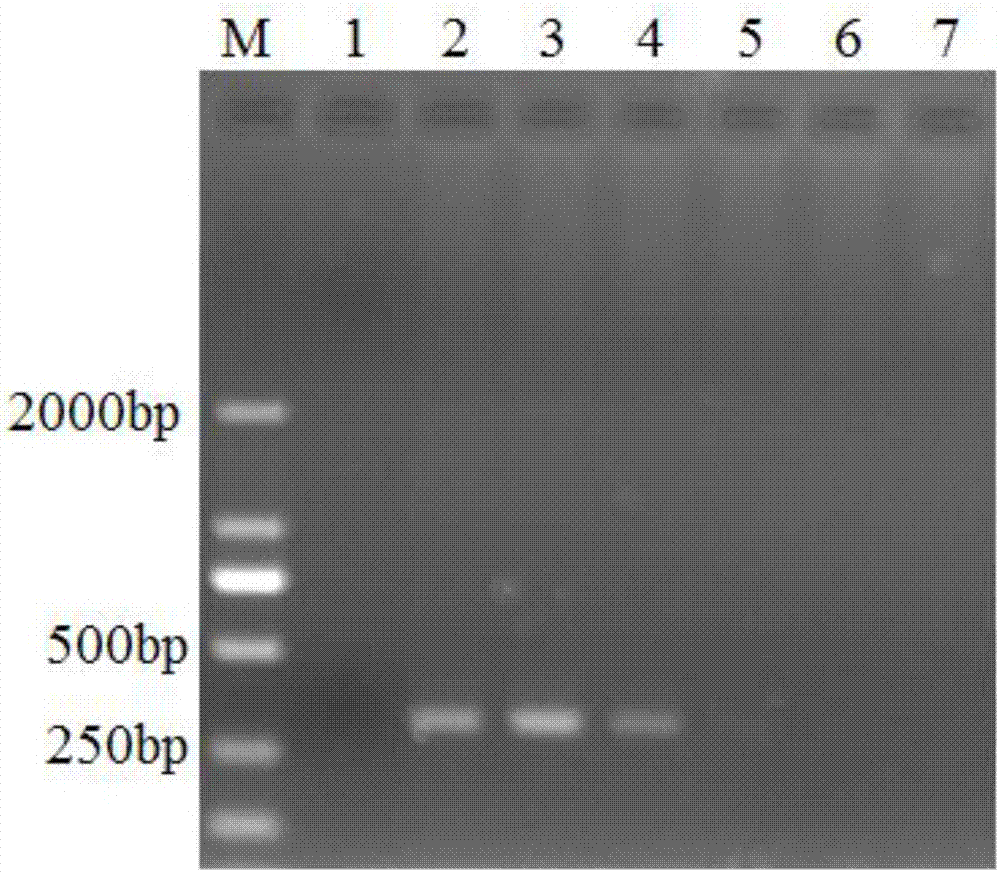
[0042] According to the RT-PCR reaction system and reaction procedure described in Example 1, the sample template RNA was replaced with foot-and-mouth disease, swine fever, pseudorabies, blue ear, and circular vaccine samples respectively, and the positive results were detected by our laboratory and identified by sequencing. The positive sample of Seneca Valley virus (i.e. named after the CH-01-2015 strain (KT321458) virus disease material RNA sample) is a positive control, and RT-PCR reaction is carried out, and the RT-PCR product is passed through 1% agarose gel Electrophoresis identification, electrophoresis results such as figure 1 As shown, the results showed that only a single band appeared at 327bp in the positive control lane, indicating that the method had good specificity.
Embodiment 3
[0043] Example 3 Sensitivity Test
[0044] According to the RT-PCR reaction system and reaction program described in embodiment one, the positive control RNA in embodiment two is used as positive control, and this positive sample RNA is pressed 10 -1 、10 -2 、10 -3 、10 -4 、10 -5 Perform 10-fold serial dilution, use distilled water as a negative control, and carry out RT-PCR reaction. The RT-PCR product is identified by 1% agarose gel electrophoresis. The electrophoresis results are as follows: figure 2 shown. The result shows that dilution 100(10 -2 ) times the band is still obvious, diluted 1000 (10 -3 ) times, there are still inconspicuous but visible bands, indicating that the method has high sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com