Seneca valley virus real-time fluorescence quantification PCR detection primer and kit
A technology for real-time fluorescence quantification and detection of primers, which is used in the determination/inspection of microorganisms, microorganisms, and microorganism-based methods, etc. It can solve the problems of indistinguishability, difficulty in diagnosis, and complicated operation methods, and achieve high accuracy and repeatability. , the effect of efficient identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of standard positive template
[0023] 1. Extraction of total viral RNA
[0024] According to the Invitrogen company TRIZOL LS Reagent RNA extraction kit instruction manual. Add 250 µL of the aliquoted virus supernatant from cell propagation and 750 µL TRIZOL to a 1.5 mL Leppendorf tube, mix thoroughly, and place at room temperature for 10 min; add 200 µL of chloroform, shake vigorously for 15 sec, and let stand at room temperature for 5 min, 4 Centrifuge at 12,000 rpm for 15 min at ℃; transfer the supernatant to a new 1.5 mL eppendorf tube, add 500 µL of isoamyl alcohol, mix well, place at room temperature for 10 min, and centrifuge at 12,000 rpm for 10 min at 4°C; discard the supernatant , precipitated with 1000 µL of ice-cold 70% ethanol, mixed gently, washed once, and centrifuged at 12,000 rpm for 10 min at 4°C; discarded the supernatant and air-dried; dissolved RNA with 20 µL of DEPC-treated triple distilled water, Store at -80°C or use dire...
Embodiment 2
[0039] Example 2 Design and Screening of Primers and TaqMan Probes
[0040] Specific primers and TaqMan probes were designed according to the 3C conserved fragments in the SVV gene sequence, and were synthesized by Huada Company. The labeled quencher is TAMRA.
[0041]
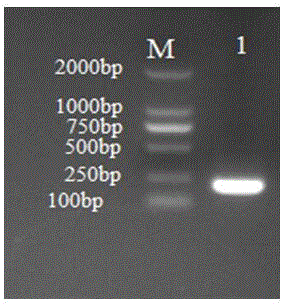
[0042] The primers and probes in Table 1 were used to perform PCR on SVV, and it was found that the other 3 pairs of primers had non-specific amplification or primer dimers, which did not meet the experimental requirements, so only primer 1 and primer 2 can be used as specific Amplify primers for detection of SVV.
Embodiment 3
[0043] Example 3 Fluorescent quantitative PCR reaction condition optimization and standard curve making
[0044] To optimize primer concentration, probe concentration and annealing temperature conditions, Real-Time PCR 20 μL reaction system is Premix Ex Taq (Probe qPCR) (2×) 10 μL, each specific upstream and downstream primers (10 μM) 0.4 μL, ROXReference Dye II (50×) 0.2 μL, fluorescent probe (10 μM) 0.8 μL, DNA template 2.0 μL, ddH 2 O 6.2 μL. The optimal reaction conditions were: 95°C for 30 s, 95°C for 5 s, 60°C for 34 s, 40 cycles.
[0045] Determination of OD of standard recombinant positive plasmid 260nm and OD 280nm value and its ratio, calculate the plasmid concentration, and convert it into copy number, serially dilute it into 7 gradients with double distilled water 10 times (10 8 ~10 3 copies / μL). Using this as a template, a 20 μL reaction system was established, and amplified on an ABI 7500 fluorescent quantitative PCR instrument according to the optimized PC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com