Promoterless cassettes for expression of alphavirus structural proteins
A technology of structural proteins and non-structural proteins, which is applied in the field of preparation of recombinant alpha virus particles, can solve the problems of lack, reduction of the theoretical number of functional recombination events, and decline in the theoretical prediction of the formation rate of replicable alpha viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
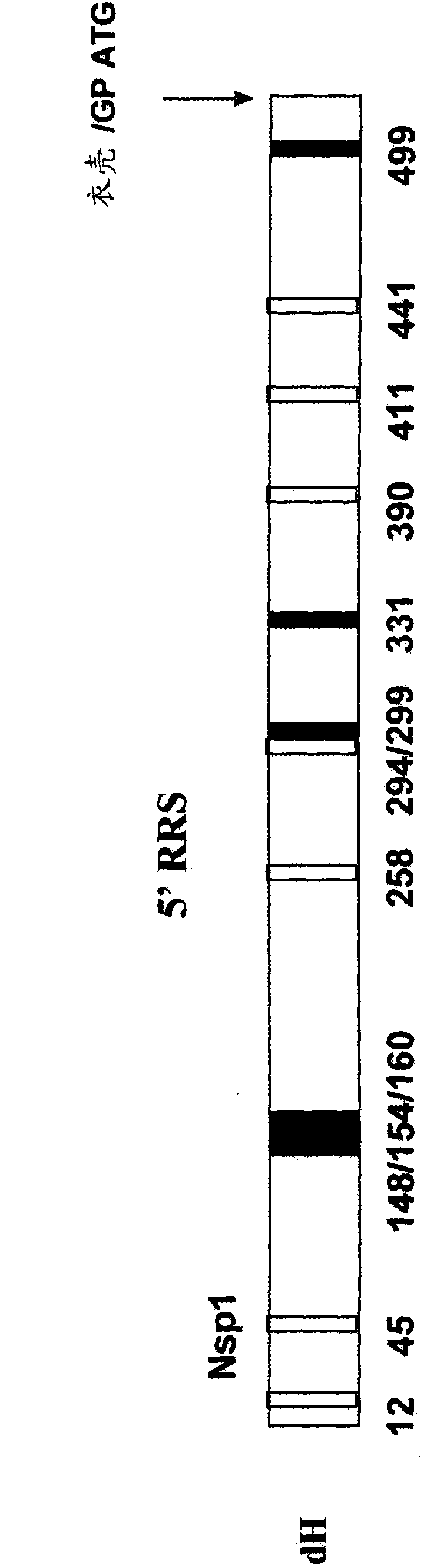
[0111] Embodiment 1: Construction of dHcap and dHgp helper
[0112] Primers (capsid F (SEQ ID NO: 98), GPF (SEQ ID NO: 60) and 13-101.pr4 (SEQ ID NO: 6) (Table 1) were designed for use from the VEE helper plasmid (for The capsid helper, referred to as "13.2.2", for the glycoprotein helper, referred to as "13.4.6") amplifies the capsid and glycoprotein (GP) genes, as described in U.S. Patent No. 5,792,462, Pushko et al. , 1997 (Virology 239:389-401) and PCT Publication WO02 / 03917 (Olmsted et al.). These primers each provide a RsrII restriction site and also bind to the beginning of the capsid or glycoprotein coding sequence. References cited above The DNA plasmids described in are a convenient source for obtaining structural protein coding fragments, e.g., by PCR amplification. Alternatively, these coding fragments can be obtained from full-length clones of VEE or attenuated variants thereof (seeing, U.S. Patent No. 5,185,440 ; U.S. Patent 5,505,947).
[0113]Amplification us...
Embodiment 2
[0115] Example 2. The expression analysis method of promoter-free auxiliary expression cassette
[0116] To determine how well the Δ26S helper constructs described here express structural proteins, each helper was electroporated into Vero cells along with the VEE replicon vector described above. To demonstrate the capability of the novel promoterless structural protein expression cassette of the present invention, the GFP or botulinum neurotoxin coding sequence was inserted into the cloning site of the VEE replicon vector. Expression of these coding sequences from particles using various combinations of the promoterless structural protein expression cassettes described herein demonstrates the utility and novelty of these cassettes.
[0117] Use RiboMAX T7 according to the manufacturer's procedure The transcription kit (Promega Corporation, Madison, WI) transcribes RNA from each helper and replicon vector by runaway transcription. Prior to electroporation, helper and replico...
Embodiment 3
[0124] Example 3: Expression analysis of full-length and truncated Δ26S helpers
[0125] The dHcap(FL) and dHgp(FL) helpers expressed protein as determined by IFA and Western blotting, and replicated efficiently as demonstrated by Northern blotting.
[0126] The complete set of truncated Δ26S replicons (deletions 1-8) for capsid and GP were analyzed, protein expression was analyzed by IFA, and how well each was expressed and replicated by Northern blotting. Each dHcap helper RNA was combined with VEE replicon RNA and 13.4.6 glycoprotein helper RNA, and the three RNAs were electroporated into Vero cells. Northern analysis and IFA were performed as described above. The results of IFA using capsid-specific antibodies are shown in Table 2. All dHcap helpers were positive for capsid expression by IFA, although the dHcap8 helper was only weakly positive.
[0127] Northern analysis of RNA extracted from electroporated cells indicated that all truncated capsid Δ26S helpers replicat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com