Monoclonal antibody for detecting SARS-CoV-2 virus nucleocapsid protein (N protein) and application thereof
A monoclonal antibody, sars-cov-2 technology, applied in the field of biomedicine, can solve the problems of high environmental, transportation, operation requirements, weak sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Recombinant SARS-Cov-2 virus N protein immunogen preparation
[0038] The complete open reading frame region was synthesized with reference to the nucleotide sequence encoding the N protein in the novel coronavirus genome sequence numbered MN908947.3 in GenBank, and cloned into the plasmid vector pUC57 (Nanjing KingScript Biotechnology Co., Ltd. company), designed the upstream primer n-Cov-NF: 5'- GGATCGAATTCGATGTCTGATAATG-3' and the downstream primer n-Cov-NR: 5'-ACGCTCGAGTTAGTGGTGGTGGTGGTGGTGGGCCTGAGTTGA GTCAGC-3', introduced the EcoRI cutting point through the upstream primer, and introduced the group through the downstream primer Amino acid purification tag and XhoI restriction site, primers were synthesized by Beijing Liuhe Huada Gene Technology Co., Ltd. According to the standard nucleic acid amplification process, the target gene was amplified from the plasmid vector, and cloned into the eukaryotic expression vector pcDNA3.4 (purchased from Invitrogen ...
Embodiment 2
[0039] Example 2 Establishment of Hybridoma Cell Lines and Antibody Screening
[0040] 1. Animal immunity
[0041] The recombinant N protein in Example 1 was inactivated at 60°C and emulsified with complete Freund's adjuvant (purchased from Sigma), and six 4-6-week-old female Balb / c mice (purchased from Beijing Weitongli Co., Ltd.) were immunized. China Experimental Animal Technology Co., Ltd.), serially numbered 64360#A, 64360#B, 64360#C, 64360#D, 64360#E and 64360#F. The dose of each mouse was injected subcutaneously in the abdomen at a dose of 60 μg / only. Immunization was boosted every 14 days, and the antigen was emulsified with Freund's incomplete adjuvant (Sigma Company) at a dose of 30 μg per mouse. 7 days after the third booster immunization, indirect ELISA (wavelength 450nm) was used to detect the polyantibody titer of the anti-immunogen in the mouse serum. The mouse with the highest titer was injected into the tail vein for shock immunization. 50μg / only.
[0042]...
Embodiment 3
[0051] Example 3 Preparation of Monoclonal Antibody by Ascites Induction Method
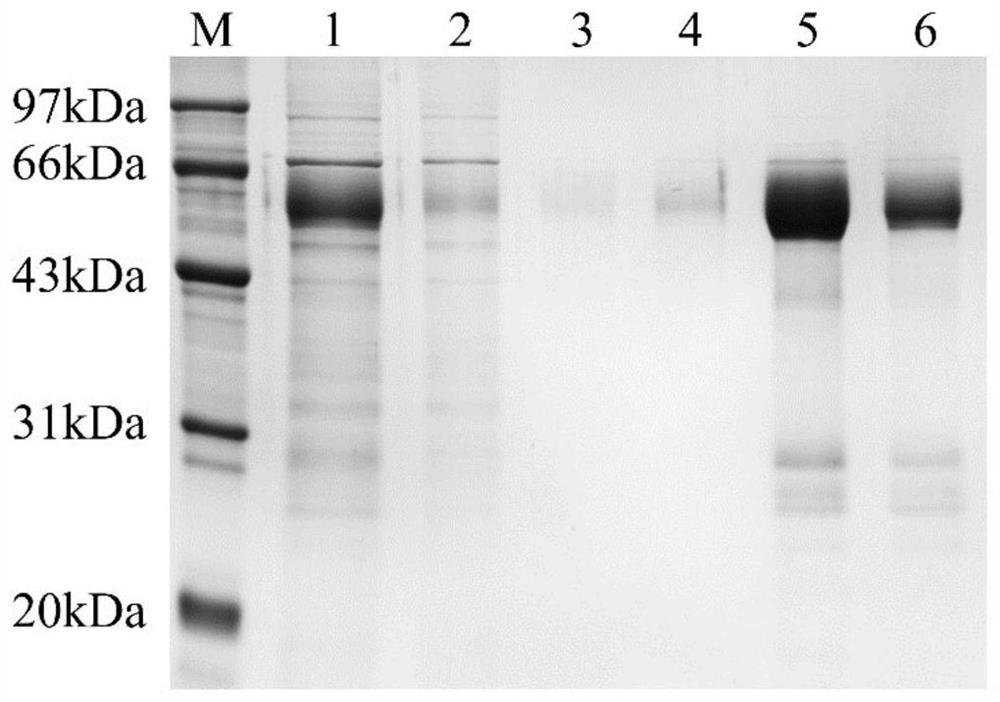
[0052] Wash and suspend the cells in the logarithmic growth phase with serum-free medium, and count about 5×10 5 , 1ml of suspended cells were injected intraperitoneally into mice sensitized with paraffin oil in advance. Ascites collection was started 7 days later. The removed ascites was centrifuged at 4000rpm at 4°C, and the ascites in the middle was carefully sucked out for 10 minutes and collected in a centrifuge tube for purification. Purification of the antibody was carried out according to the instructions of HiTrap rProtein AFF (purchased from GE, catalog number 17-5079-02).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com