Protein delivery system
A technology of protein and protein fusion, which is applied in the field of delivery of protein substances to cells, and can solve problems such as the difficulty of delivering proteins to cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
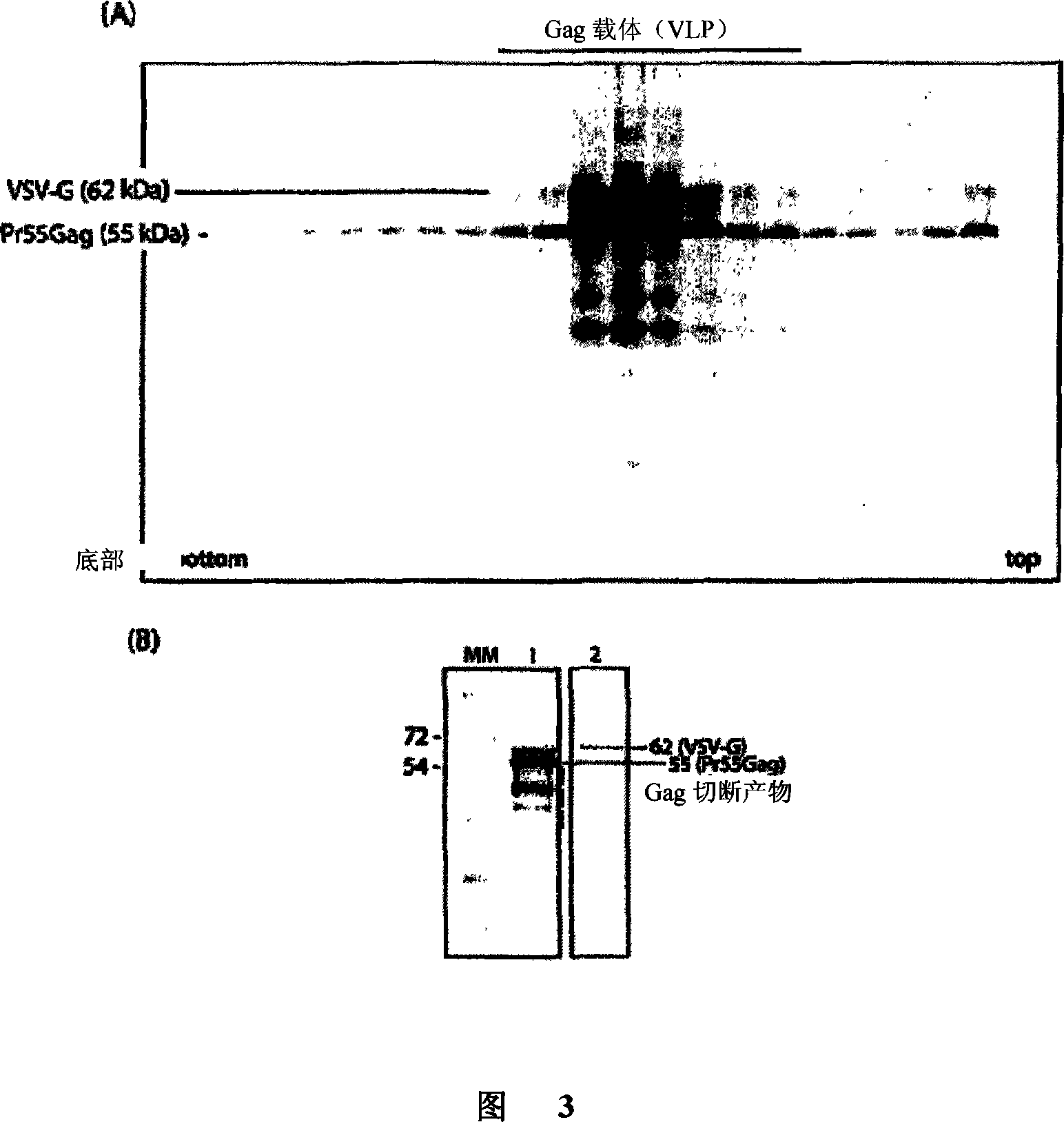
[0134] Example 1: Fabrication of CFTR-GFP VLPs
[0135] A chimeric gene encoding green fluorescent protein (GFP) fused to CFTR with a GFP domain at the N-terminus of the CFTR protein was incorporated into the VLPs. The GFP moiety-tagged CFTR enables rapid recognition and enables rapid establishment of the tagged protein. The fusion gene GFP-CFTR was cloned in two different expression systems, (i) baculovirus AcMNPV to form recombinant baculovirus AcMNPV-GFPCFTR, (ii) in adenovirus type 5 (Ad 5) to produce recombinant virus Ad 5-GFP-CFTR. The protein was observed to localize to the plasma membrane of the cell. Similar GFP-tagged structures carried by expression plasmids have been reported to have the same function as the non-tagged protein CFTR (Haggie et al., 2002, J. Biol. Chem. 277:16419-16425 and Loffing-Cueni et al., 2001, Am. J .Physiol.Cell Physiol.281:C1889-1897.20).GFP-CFTR clones were shown to be activated and functional (Robert et al., 2004, J.Biol.Chem.279:21160-...
Embodiment 2
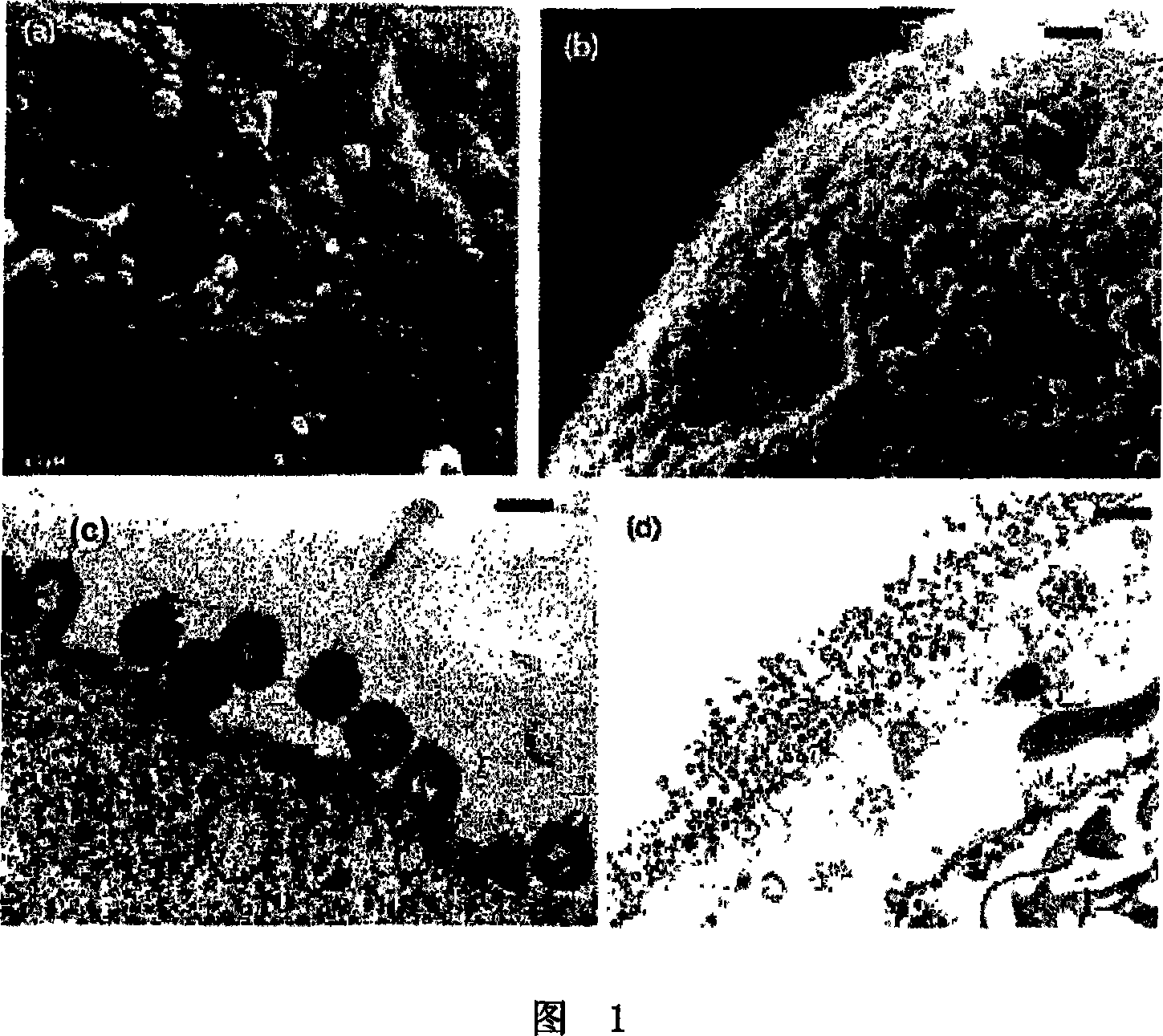
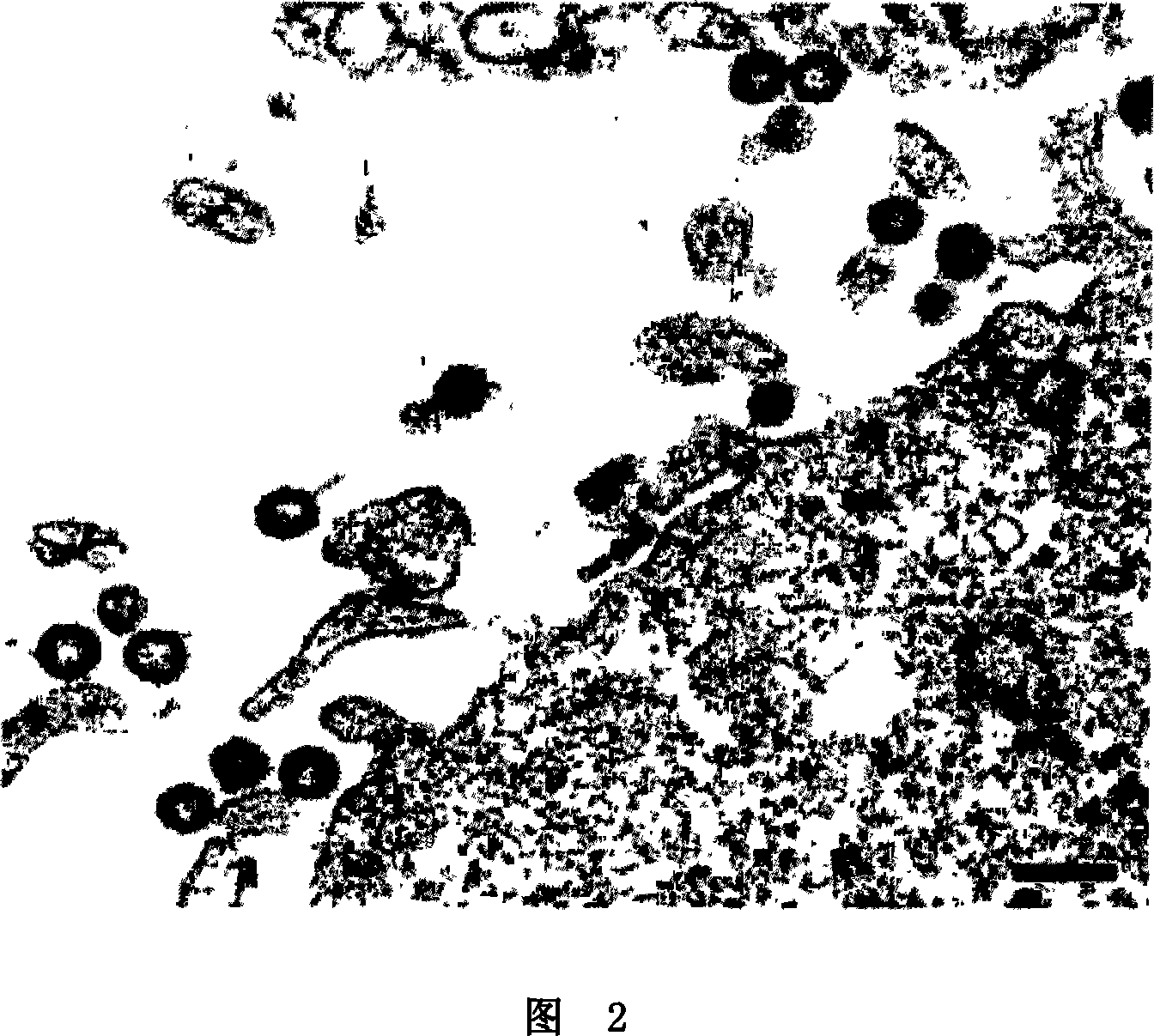
[0140] Example 2: Transmission Electron Microscopy
[0141] Cells were infected with the indicated viruses at a multiplicity of infection (MO1) of 25 as indicated in the legend. Cells were collected for electron microscopy (EM) examination by pelleting by centrifugation 48 hours post-infection. Cell pellets were then fixed with 2.5% glutaraldehyde in 0.1 mpH 7.5 phosphate buffer, post-fixed with osmium tetroxicle, and treated with tannic acid (0.5% in H2O). After dehydration, samples were embedded in epoxy resin (Epon) (Epon-812, Fulham, Latham, New York). Sections were extracted, stained with 2.6% basic lead citrate-0.5% Sections uranyl acetate in 50% ethanol, and examined under a Jeol 1200-EX electron microscope.
Embodiment 3
[0142] Example 3: Scanning Electron Microscopy
[0143] Cells were grown on glass slides and infected at a multiplicity of infection (MOI) of 25 with the viruses indicated. Fixation and postfixation were the same as described for transmission EM. Cells were then point dried with liquid CO2, covered with gold, and examined with a Zeiss DSM 950 electron microscope.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com