Prevention, treatment and detection of pig progressive atrophic rhinitis
A technology for atrophic rhinitis and vaccines, which can be applied to antibody medical components, medical preparations containing active ingredients, bacterial antigen components, etc., and can solve the problems of tediousness, high operation time and cost, and time-consuming steps of culturing cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1. Molecular-level identification of Type A and Type D Pasteurella multocida and nucleotide sequence alignment of PMT-encoding genes
[0120] I. Materials and methods:
[0121] A, strain source:
[0122]The applicant took the nursery pigs culled from the monitoring pig farms in the four counties and cities of Taichung City, Taichung County, Changhua County and Nantou County in central Taiwan as the object to carry out the epidemic investigation, sampling and pathological examination of porcine atrophic rhinitis. Among the isolates biochemically identified as Pasteurella multocida, D-type toxigenic strains (PMD 14, PMD 21, PMD 48) and D-type non-toxigenic strains (PMD) were selected by the CPE effect test of Vero cells. 20, PMD 27), A-type toxigenic strains (PMA 2, PMA 10, PMA 23) and A-type non-toxigenic strains (PMA 33, PMA 59), a total of 10 isolates, and additionally used standard reference strains (D type The toxigenic strain NCTC 12178 was purchased from ...
Embodiment 2
[0176] Example 2. Establishment of DNA clones encoding PMT subunits
[0177] A. Cloning of PMT-encoding gene:
[0178] PCR reaction of PMT-encoding gene:
[0179] According to item 1 in Example 1, item F in materials and methods, extraction of chromosomal DNA and determination of concentration, the total DNA extract was obtained from the D-type Pasteurella multocida PMD-48 strain culture, and used Absorbance A 260 / 280 Determine the concentration and purity of DNA.
[0180] In the cloning of the PMT-encoding gene, PCR was performed using the above-obtained DNA total extract as a template and a set of primer pairs A1B2 shown in Table 1:
[0181] forward primer
[0182] A1: 5′-agaggttat ggatcc gaaaacaaaacatttt-3' (SEQ ID NO: 3)
[0183] BamHI
[0184] reverse primer
[0185] B2: 5′-ctcttgttaa gctagc ctttgtgaaaagaggag-3' (SEQ ID NO: 10)
[0186] NheI
[0187] Since there is no BamHI and NheI restriction enzyme cleavage sites in the P...
Embodiment 3
[0217] Example 3. Toxicity test of PMT recombinant subunit (rsPMT)
[0218] Vero Cytotoxicity Assay:
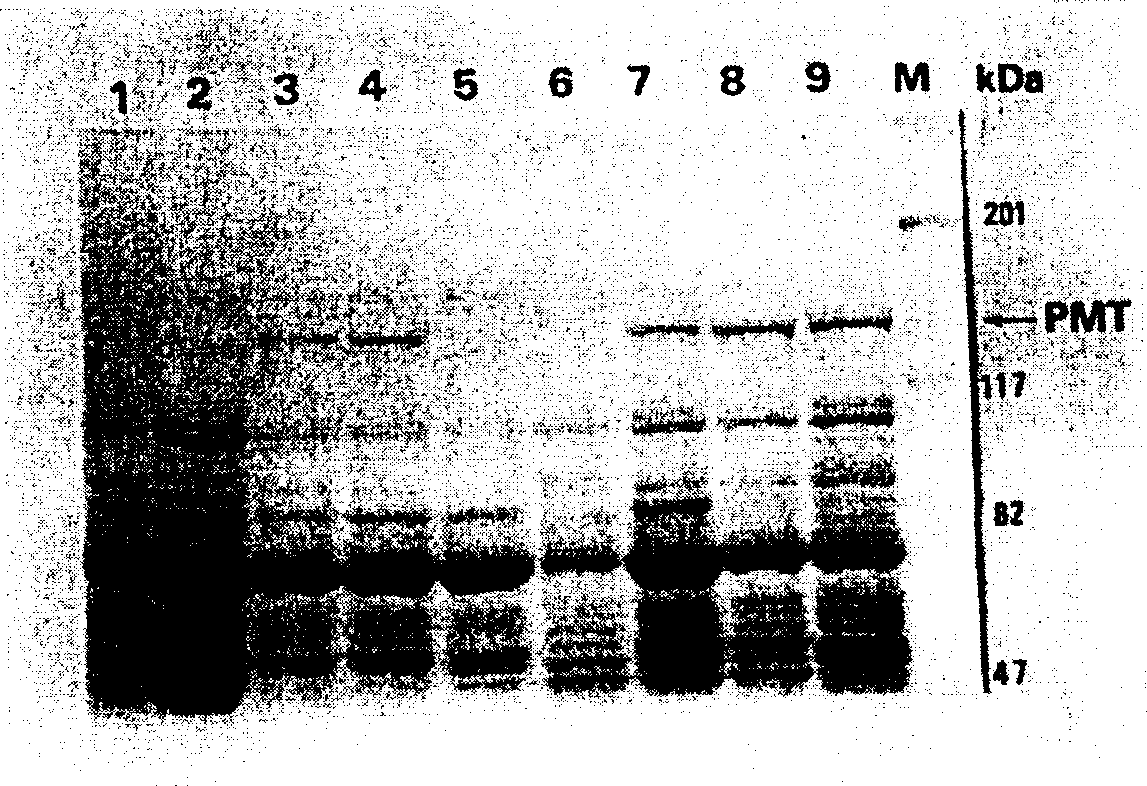
[0219] The rsPMT cloned strains induced by IPTG were centrifuged at 5,000×g for 10 minutes to collect bacteria, washed repeatedly with sterile PBS to suspend the bacteria, and sonicated (output power 20% for 30 minutes; MisonixXL2020, Heat Systems) Inc.) crushed the bacteria followed by high speed centrifugation at 10,000 xg for 30 minutes. Take the supernatant and filter it through 0.45 μm and 0.22 μm pore size filter membranes. The filtrate filtered twice is the crude soluble protein of the expression product, and the Vero cytotoxicity test is carried out. The experimental method is as in Example 1. I. Item D in Materials and Methods, Determination of cytopathic effect (CPE) of PMT protein on Vero cell line of African green ape. The results of the Vero cytotoxicity assay are shown in Figures 15-17.
[0220]In this experiment, the crude PMT toxin protein expressed by Past...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com