Prevention, treatment and detection of progressive atrophic rhinitis of pig
A technique for atrophic rhinitis and musculocidal, applied in the direction of antibody medical ingredients, medical preparations containing active ingredients, bacterial antigen ingredients, etc., can solve the problems of high operating time and cost, cumbersome, time-consuming steps for culturing cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Embodiment 1.Molecular-level identification of type A and D type Pasteurella multocida (Pasteurella multocida) and the nucleotide sequence alignment of the PMT encoding gene
[0121] I. Materials and methods:
[0122] A. Source of the strain:
[0123] The applicant conducted investigation, sampling and pathological examination of swine atrophic rhinitis on pigs eliminated from pig farms in Taichung City, Taichung County, Changhua County, and Nantou County in central Taiwan. Among the isolates identified as Pasteurella multocida biochemically, D-type toxin-producing strains (PMD 14, PMD 21, PMD 48), D-type non-toxin-producing strains (PMD 20, PMD 27), A-type toxin-producing strains (PMA 2, PMA 10, PMA 23) and A-type non-toxin-producing strains (PMA 33, PMA 59), a total of 10 isolates, and additional standard reference strains (D-type The toxin-producing strain NCTC 12178 was purchased from the Culture Center of the Food Industry Development Institute, and the type A no...
Embodiment 2
[0179] Example 2. Establishment of DNA clones encoding PMT subunits
[0180] A, the cloning of PMT coding gene:
[0181] PCR reaction for PMT-encoding gene:
[0182] According to the extraction and concentration determination of I in embodiment 1, the F item in the material and method, chromosomal DNA, and obtain DNA total extract from D type Pasteurella multocida PMD-48 bacterial strain culture, and with Absorbance A 260 / 280 Determine the concentration and purity of DNA.
[0183] When carrying out the cloning of PMT coding gene, take the DNA total extract obtained above as template, and use a group of primer pair A1B2 shown in Table 1 to carry out PCR:
[0184] forward primer
[0185] A1: 5′-agaggttat ggatcc gaaaacaaaacatttt-3' (SEQ ID NO: 3)
[0186] BamHI
[0187] reverse primer
[0188] B2: 5′-ctcttgttaa gctagc ctttgtgaaaagaggag-3' (SEQ ID NO: 10)
[0189] NheI
[0190] Since there are no restriction enzyme cleavage sites of BamHI and NheI in the PMT coding ge...
Embodiment 3
[0221] The toxicity test of embodiment 3.PMT recombinant subunit (rsPMT)
[0222] Vero cytotoxicity test:
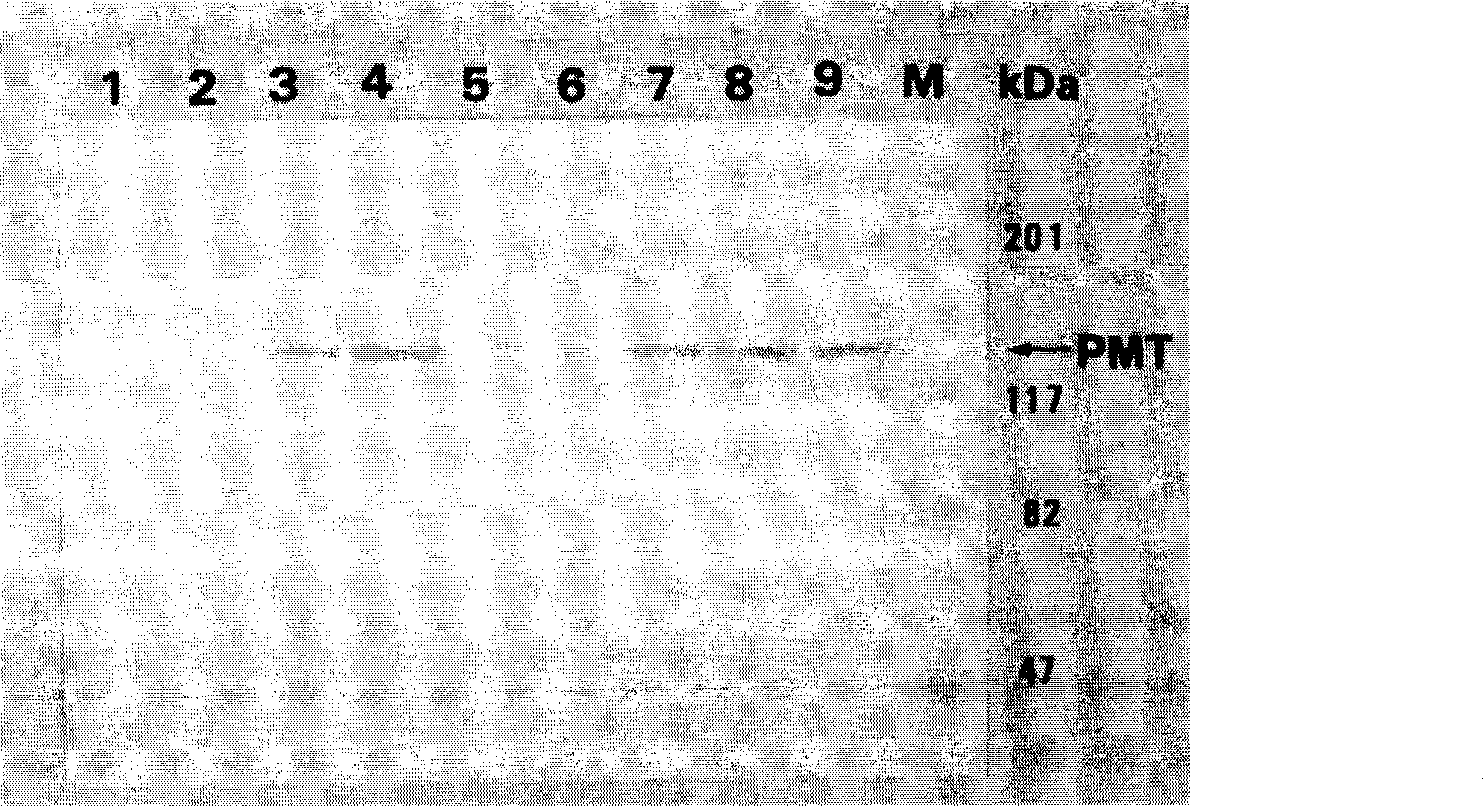
[0223] The rsPMT clone strain induced by IPTG was centrifuged at 5,000xg for 10 minutes to collect the bacteria, then washed repeatedly with sterile PBS to suspend the bacteria, and ultrasonic (20% output power, 30 minutes; MisonixXL2020, Heat Systems Inc.) to crush the bacteria followed by high speed centrifugation at 10,000 xg for 30 minutes. Take the supernatant and filter it through filter membranes with a pore size of 0.45 μm and 0.22 μm. The filtrate filtered twice is the crude soluble protein of the expression product, and the Vero cell toxicity test is carried out in this way. The experimental method is according to Example 1. Item I, item D in materials and methods, determination of cytopathic effect (cytopathic effect, CPE) of PMT protein on African green ape kidney cell line (Vero cell). Vero cytotoxicity assay results are shown in Figures 15-17.
[0224] I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com