Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
A technology of inactivated vaccines and multivalent vaccines, which can be used in medical preparations containing active ingredients, biochemical equipment and methods, microorganisms, etc., and can solve the problem of not finding prevention and treatment drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
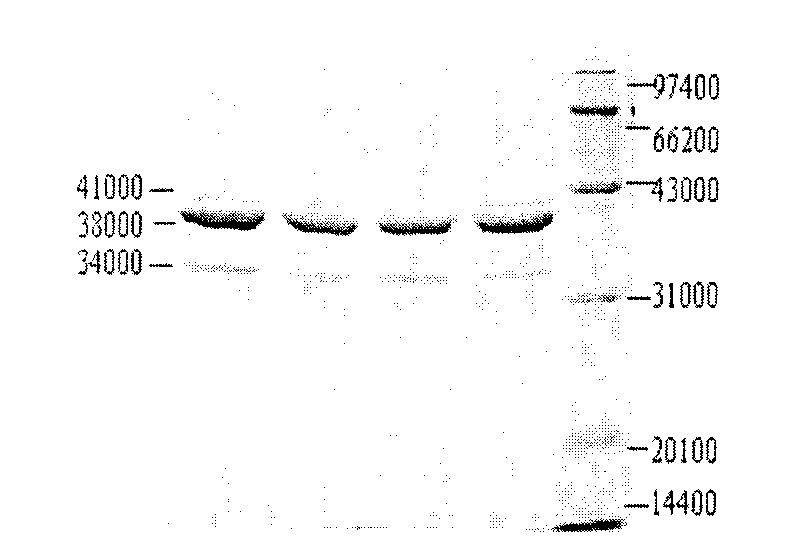
Image
Examples
Embodiment 1
[0130] Example 1: Chicken embryo culture to obtain influenza pentavalent vaccine antigen
[0131] Seasonal influenza (H1N1, H3N2, type B), highly pathogenic H5N1 human avian influenza, and influenza A H1N1 influenza virus vaccine strains are provided by the US CDC and the UK NIBSC.
[0132] Inoculate seasonal influenza (H1N1, H3N2, type B), highly pathogenic H5N1 human avian influenza and influenza A H1N1 influenza virus vaccine strains into the allantoic cavity of 9-11-day-old SPF chicken embryos, and culture them at 33-35°C for 48-72h The chicken embryos were frozen overnight in a refrigerator at 4°C, and the allantoic fluid of the chicken embryos was harvested to obtain a large amount of virus. After the viruses were harvested, they were filtered through a filter with a pore size of 0.45 μm to obtain the influenza pentavalent vaccine antigen.
Embodiment 2
[0133] Example 2: Chicken embryo culture to obtain influenza multivalent vaccine antigen
[0134] Seasonal influenza (H1N1, H3N2, type B), highly pathogenic H5N1 human avian influenza, influenza A H1N1 and other subtype influenza virus strains were provided by CDC in the United States and NIBSC in the United Kingdom.
[0135] Inoculate the allantoic cavity of 9-11-day-old SPF chicken embryos with seasonal influenza (H1N1, H3N2, B-type), highly pathogenic H5N1 human avian influenza, A-H1N1 influenza and other subtype influenza virus strains, at 33-35°C Cultivate for 48-72 hours, freeze the chicken embryos overnight in a 4°C refrigerator, harvest the allantoic fluid of the chicken embryos to obtain a large amount of virus, and after the virus is harvested, pass it through a filter for clarification and filtration to obtain influenza or multivalent vaccine antigens.
Embodiment 3
[0136] Example 3: Mammalian cells (such as Vero cells, MDCK cells, Per.C6 cells, 2BS cells, etc.) were cultured to obtain influenza pentavalent vaccine antigens
[0137] Seasonal influenza (H1N1, H3N2, type B), highly pathogenic H5N1 human avian influenza, and influenza A H1N1 influenza virus vaccine strains are provided by the US CDC and the UK NIBSC.
[0138] Cultured cells: Mammalian cell lines used for vaccine production (such as Vero cells, MDCK cells, Per.C6 cells, 2BS cells, etc.) were planted in 15L spinner bottles at a ratio of 1:6, cultured for 2-3 days and then inoculated with influenza virus vaccines Strains, including seasonal influenza (H1N1, H3N2, type B), highly pathogenic H5N1 human avian influenza and influenza A (H1N1) influenza virus vaccine strains; wherein the cells are recovered from the working generation cell bank and obtained through passage amplification;
[0139] Inoculation of virus: at cell density up to 3 x 10 6 Inoculate the virus with a multip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com