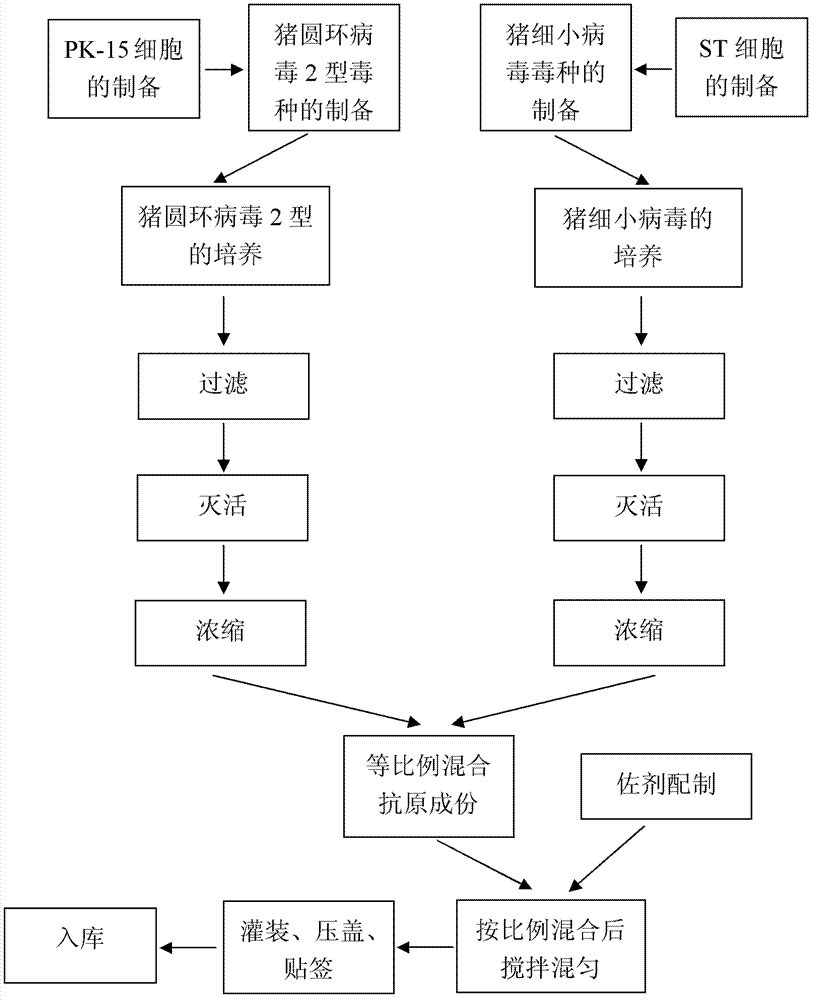
Bivalent inactivated vaccine of porcine circovirus type 2 and porcine parvovirus and preparation method thereof
A dual inactivated vaccine and porcine circovirus technology, which is applied in the direction of antiviral agents, virus antigen components, pharmaceutical formulations, etc., can solve the problems of failure to develop the dual vaccine, difficulty in control, mutual interference, etc., and achieve immunity Convenient and quick, reduce side effects, improve survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
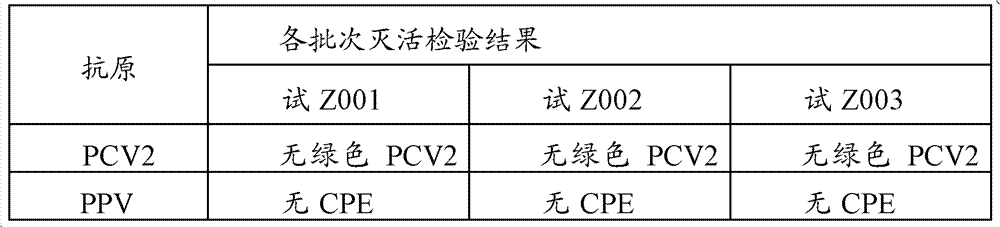
[0044] The preparation and inspection of the dual inactivated vaccine of embodiment 1PCV2, PPV
[0045] 1.1 Preparation of poisonous seeds for production
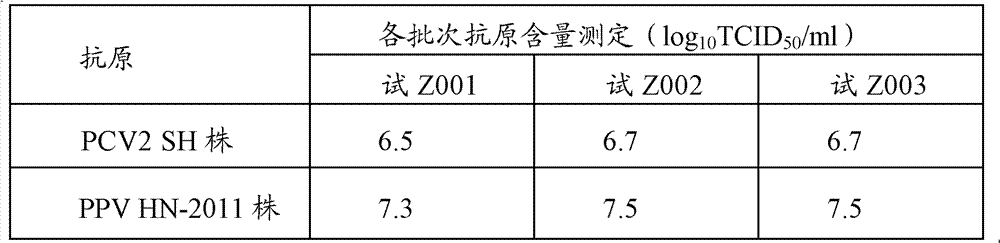
[0046] Preparation of PCV2SH strain: Dilute the virus seeds appropriately with the virus diluent (serum-free MEM medium), inoculate them on PK-15 cells (CCTCC, No. GDC0060) at a multiplicity of infection (M.O.I.) of 0.01, and incubate at 37°C for 30 minutes , add MEM cell maintenance solution containing 4% (v / v) calf serum and 2mmol / L D-glucosamine hydrochloride, culture at 37°C for 4 days, freeze and thaw 2 to 3 times, harvest virus, virus titer ≥ 10 6.5 TCID 50 / ml.
[0047] Preparation of PPV HN-2011 strain: Dilute the virus seeds appropriately with virus diluent (serum-free α-MEM medium), inoculate them in ST cells (CCTCC, number GDC0007) at a multiplicity of infection (M.O.I.) of 0.01, and cultivate them for 37 ℃ for 30 min, add MEM cell maintenance solution containing 1% (v / v) calf serum and 2 mmol / L D-glucosamine ...
Embodiment 2
[0100] Example 2 PCV2, PPV dual inactivated vaccine and the comparison of the immune effect of using two kinds of vaccines (PPV inactivated vaccine and PCV2 inactivated vaccine) to immunize piglets alone
[0101] 1. Materials
[0102] PCV2, PPV double inactivated vaccine, select the laboratory product among the embodiment 1 for use (batch number is trial Z001); PCV2 inactivated vaccine (SH strain), Pulaike Biological Engineering Co., Ltd. produces (batch number is 100903), virus The content is at least 10% before inactivation 6.5 TCID 50 / ml; PPV disease inactivated vaccine, Wuhan Zhongbo Biological Co., Ltd. (batch number is 101001), the virus content is at least 10% before inactivation 7.5 TCID 50 / ml.
[0103] 2. Design of animal experiments
[0104] 80 weaned piglets aged 21-28 days were selected and divided into 4 groups, 20 pigs in each group; each pig in the first group was injected with 2ml of PCV2 and PPV dual inactivated vaccine (batch number 001) in the neck mu...
Embodiment 3
[0110] Reproductive performance and growth of piglets after embodiment 3 PCV2, PPV dual inactivated vaccine immunization sow
[0111] 1. Materials
[0112] PCV2, PPV double inactivated vaccine, select the laboratory product among the embodiment 1 for use (lot number is trial Z001). PCV2 inactivated vaccine (SH strain), produced by Pulaike Bioengineering Co., Ltd. (batch number 100903); PPV disease inactivated vaccine, Wuhan Zhongbo Biological Co., Ltd. (batch number 101001).
[0113] 2. Design of animal experiments
[0114] Select 60 gilts aged 6-7 months and divide them into 3 groups with 20 gilts in each group. In the first group, each gilt was immunized with dual inactivated vaccine one month before mating, boosted 1 time after 14 days, and was immunized for the third time 1 month before delivery, with 2ml / head each time. In the second group, each gilt was immunized with PCV2 and PPV disease inactivated single vaccine at the same time 1 month before mating, and boosted i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com