Recombinant multivalent vaccine
A virus genome and varicella zoster technology, applied in the field of recombinant varicella zoster virus, can solve the problem of inserting multiple antigens into the virus genome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] (Example 1: Selection of a region (gene) to be inserted into a BAC vector)
[0331] When using BAC vectors to prepare recombinant viruses inserted with foreign antigen genes, the genome size increases due to the insertion of many foreign genes. It is known that if the genome size is too large, the genomic DNA cannot be packaged into the capsid, and as a result, recombinant virus cannot be prepared. Therefore, in order to insert most of the antigen genes into the Oka vaccine strain, knockout of non-essential genes of the Oka vaccine strain must be considered to reduce the size of the genomic DNA. In addition, a non-essential gene that can grow even if the virus is knocked out is suitable as a site for inserting a foreign sequence such as a BAC vector sequence or a gene encoding an antigenic protein derived from another virus.
[0332] Therefore, using the recombinant DNA (P-Oka strain VZV-BAC-DNA) obtained by inserting the genome of the original strain of varicella viru...
Embodiment 2
[0339] (Example 2: Preparation of a multivalent vaccine by inserting the mumps virus HN gene into the ORF of gene 13)
[0340] As shown in Example 1, gene 13 is a suitable gene for knockout and / or insertion of foreign sequences, therefore, a multivalent vaccine is prepared by inserting the mumps virus HN gene into the ORF of gene 13.
[0341] The HN gene and F gene of mumps virus were amplified from the wild strain-Iwasaki strain using PCR. Analyzing the amino acid sequences of the F gene and HN gene of the cloned Iwasaki strain, the F gene showed higher homology (>98.5) compared with the field strain or the vaccine strain, while the HN gene had a higher homology (>98.5) with the field strain after the late 1990s. The homology of the strains is high, and the homology with field strains or vaccine strains before the early 1990s is low (about 96%).
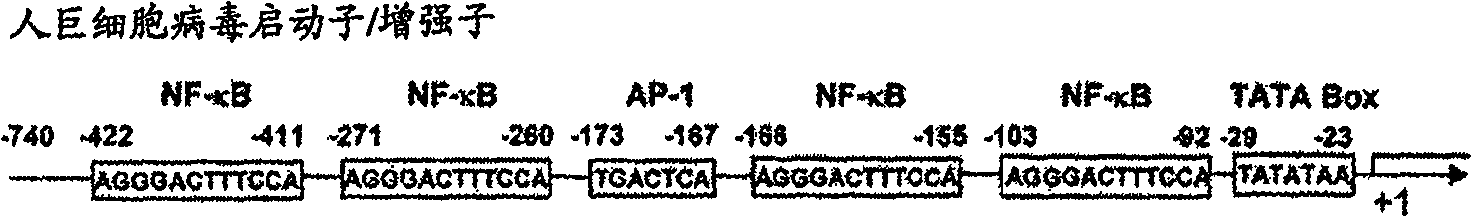
[0342] Next, a human cytomegalovirus (CMV) promoter / enhancer sequence was operably linked upstream of the cloned gene. The plasm...
Embodiment 3
[0349] (Preparation of mutant recombinant varicella-zoster virus strain with weak pathogenicity)
[0350] According to the present invention, a mutant recombinant varicella-zoster virus can be prepared by adopting the following method, and a mutant varicella-zoster virus strain with weak pathogenicity can be obtained from the mutant virus.
[0351] (1: Preparation of mutant recombinant varicella-zoster virus)
[0352] The preparation method of the mutated recombinant varicella-zoster virus includes, for example: preparing the mutated recombinant varicella-zoster virus by homologous recombination between the nucleic acid containing the mutated gene and the VZV-BAC-DNA plasmid. The mutated gene used in homologous recombination with the VZV-BAC-DNA plasmid may have random variation or directed site variation. Using any one of the above methods, a mutant recombinant varicella-zoster virus group with random variation and a mutant recombinant varicella-zoster virus with directed si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com