Varicella-herpes zoster mRNA vaccine composition, and preparation method and application thereof
A vaccine composition, herpes zoster technology, applied in the field of vaccines, can solve the problems of difficult quality control in the preparation process, stability needs to be further improved, active ingredients are sensitive to temperature, etc., to save time and economic costs, and avoid genome integration risk, the effect of enhancing antigen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1——mRNA preparation:
[0070]Encoding the full-length amino acid sequence of varicella-zoster virus Oka strain glycoprotein E (UniProtKB / Swiss-Prot: Q9J3M8.1, see SEQ ID No.1), the amino acid sequence of mutations introduced into the carboxy-terminal intracellular region (Y569A, S593A, S595A, T596A, T598A, see SEQ ID No.2), the gene containing only the amino acid sequence of the extracellular region (see SEQ ID No.3) is optimized according to mammalian codon bias, together with the 5' untranslated region sequence, 3 The 'untranslated region sequence and the 3' end polyadenylation sequence were synthesized by Shanghai Sangong, and constructed between the XhoI and BamHI restriction sites on the plasmid pBlueScript II SK(+). After linearization, the mRNA synthesis kit was used (purchased from Shanghai Lanque Biology) was transcribed in vitro by co-transcription chemical substrate capping, and further purified using RNA purification kit (purchased from NEB). Qua...
Embodiment 2
[0071] Embodiment 2——Preparation of varicella-zoster mRNA vaccine composition
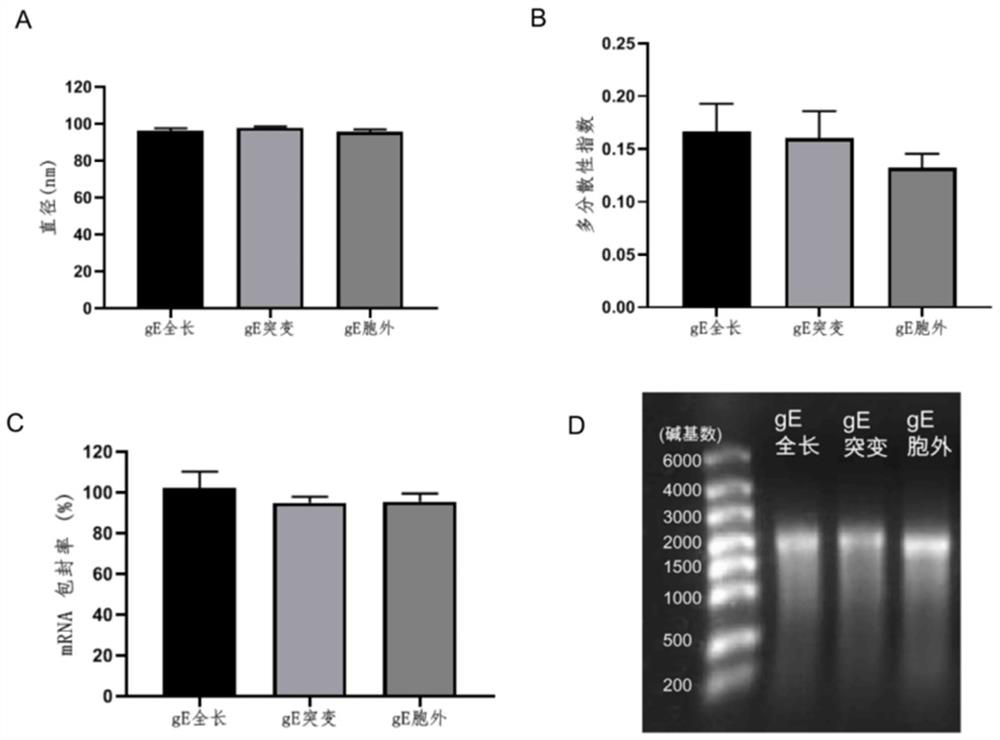
[0072] According to MC3: DSPC: cholesterol: DMG-PEG2000 molar ratio of 50:10:37.5:2.5, weigh the lipid (purchased from Shanghai Aiweituo) and dissolve it in absolute ethanol to form solution A; prepare 0.1 mg of Example 1 The mRNA was dissolved in 100mM, pH 4.0 citrate buffer to form solution B; use a microfluidic nanomedicine manufacturing system (NanoAssemblr Ignite from Precision Nanosystems, Canada) to mix solution A: solution B at a volume ratio of 1:3, and use 40 times the volume The varicella-zoster mRNA vaccine composition is obtained after dialysis with PBS buffer solution and sterilization through a 0.22 micron filter membrane.
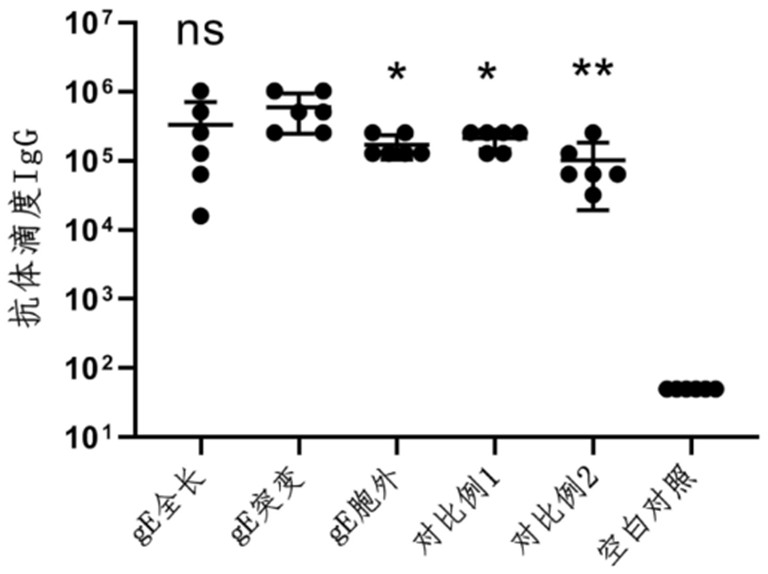
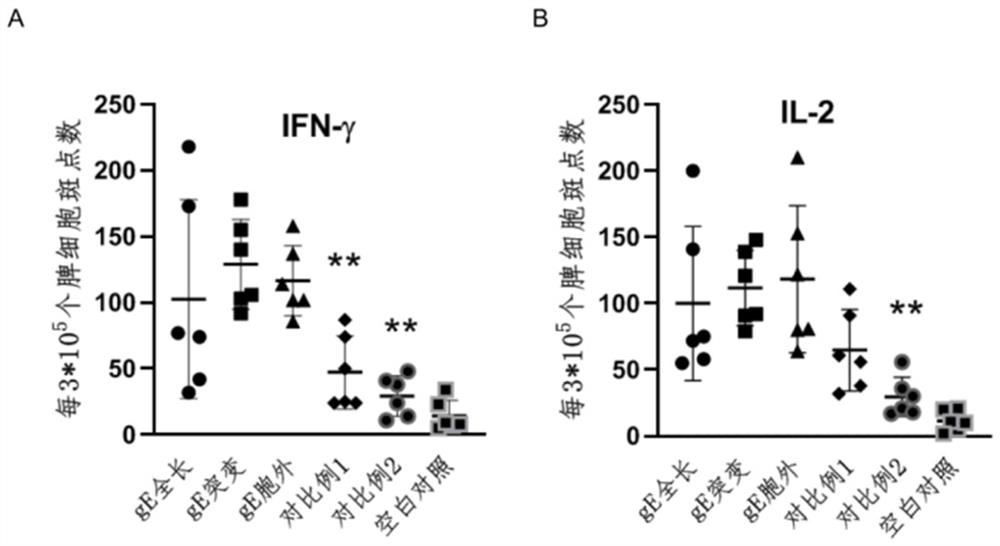
[0073] For each mRNA vaccine composition prepared in the above embodiment 2, the following measurements were carried out:
[0074] 1. Particle size and polydispersity index
[0075] The particle size and polydispersity index of LNP were measured using Zetasizer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com