Protein recombinant lactococcus lactis for secretory expression of core antigen COE of PEDV (Porcine Epidemic Diarrhea Virus) as well as preparation method and application of protein recombinant lactococcus lactis
A Lactococcus lactis, core antigen technology, applied in the field of animal biomedical engineering, can solve problems such as poor immune effect, and achieve the effect of promoting healthy development and reducing harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
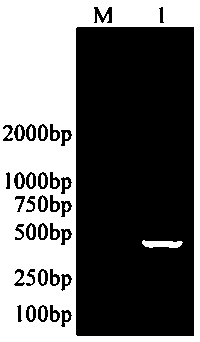
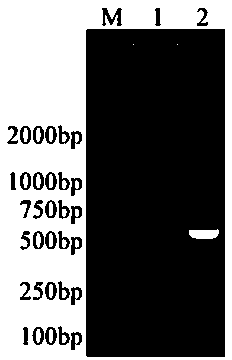
Image
Examples
Embodiment 1
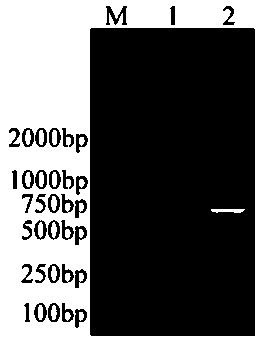
[0051] Example 1 Construction of secreted recombinant Lactococcus lactis containing COE gene
[0052] S1. Construction of secretory recombinant plasmid pNZ8048-COE
[0053] S11. Codon preference optimization and synthesis of related gene sequences: according to the sequence of the target gene PEDV COE gene and the characteristics of the expression vector pNZ8048, as well as the signal peptide sequence added for the purpose of efficient secretion and expression SP Usp45 And the enhanced sequence [(amino acid sequence is: LEISSTCDA, the insertion position is after the Usp45 signal peptide, before the target gene COE, in the middle, and the positional relationship is: ( PnisA:: SP Usp45 :: LEISSTCDA :: COE ]), and the His (histidine) tag sequence added for easy detection, the entire expected expression sequence is available on the dedicated website ( http: / / genomes.urv.es / OPTIMIZER ) to optimize the codon preference for expression of lactic acid bacteria, and send the 42...
Embodiment 2
[0064] Application of embodiment 2 Lactococcus lactis in the preparation of PEDV vaccine
[0065] Prepared by Example 1 L. lactis NZ9000 / pNZ8048-COE recombinant Lactococcus lactis is made into an oral vaccine for preventing porcine epidemic diarrhea, and the immune effect of the vaccine is studied, including the following steps:
[0066] S1. L. lactis Preparation of NZ9000 / pNZ8048-COE recombinant Lactococcus lactis oral vaccine: the L. lactis NZ9000 / pNZ8048-COE recombinant expression bacteria were inoculated in G-M17C liquid medium at a ratio of 1:100, cultured overnight at 30°C, and the overnight culture was inoculated in 10 mL of G-M17 liquid medium at a ratio of 1:50. Continue to culture for about 2.5 hours until the bacteria enter the logarithmic growth phase, add 1 μg / mL inducer (Nisin) to a final concentration of 2 ng / mL, stop the culture after 6 hours of induction, and the whole culture at this time is directly used as an oral vaccine , using the gradient diluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com