Method for detecting pathogenic microorganism by using antigen-stimulated cellular immune response and test pen
A technology of pathogenic microorganisms and cellular immunity, applied in the fields of immunology, protein detection and molecular biology, can solve the problems of delayed treatment time, low content of pathogenic microorganisms, complicated process, etc., to improve specificity and sensitivity, reduce manpower and material resources, simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the preparation of the colloidal gold immunochromatography test pen that detects IFN-γ
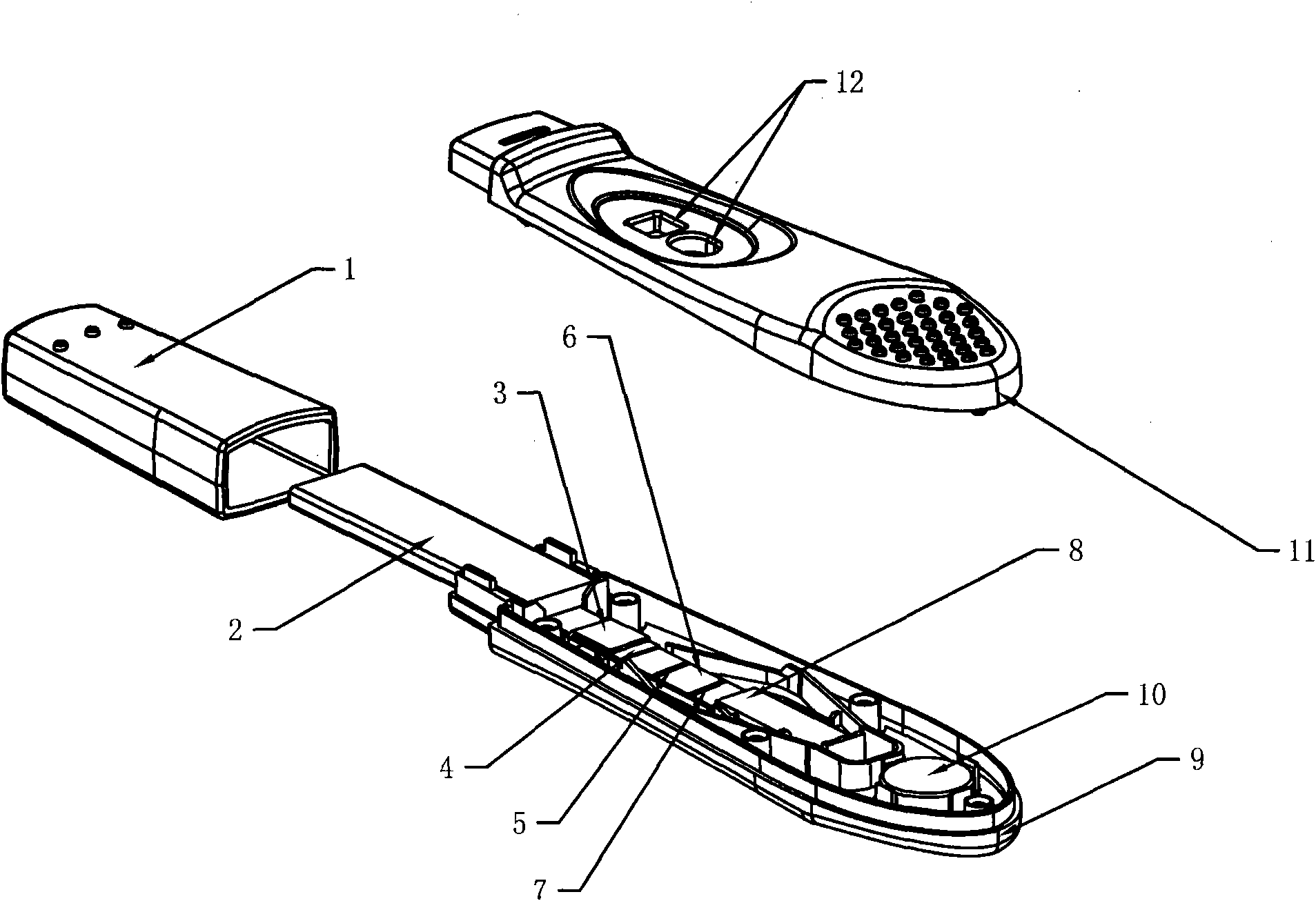
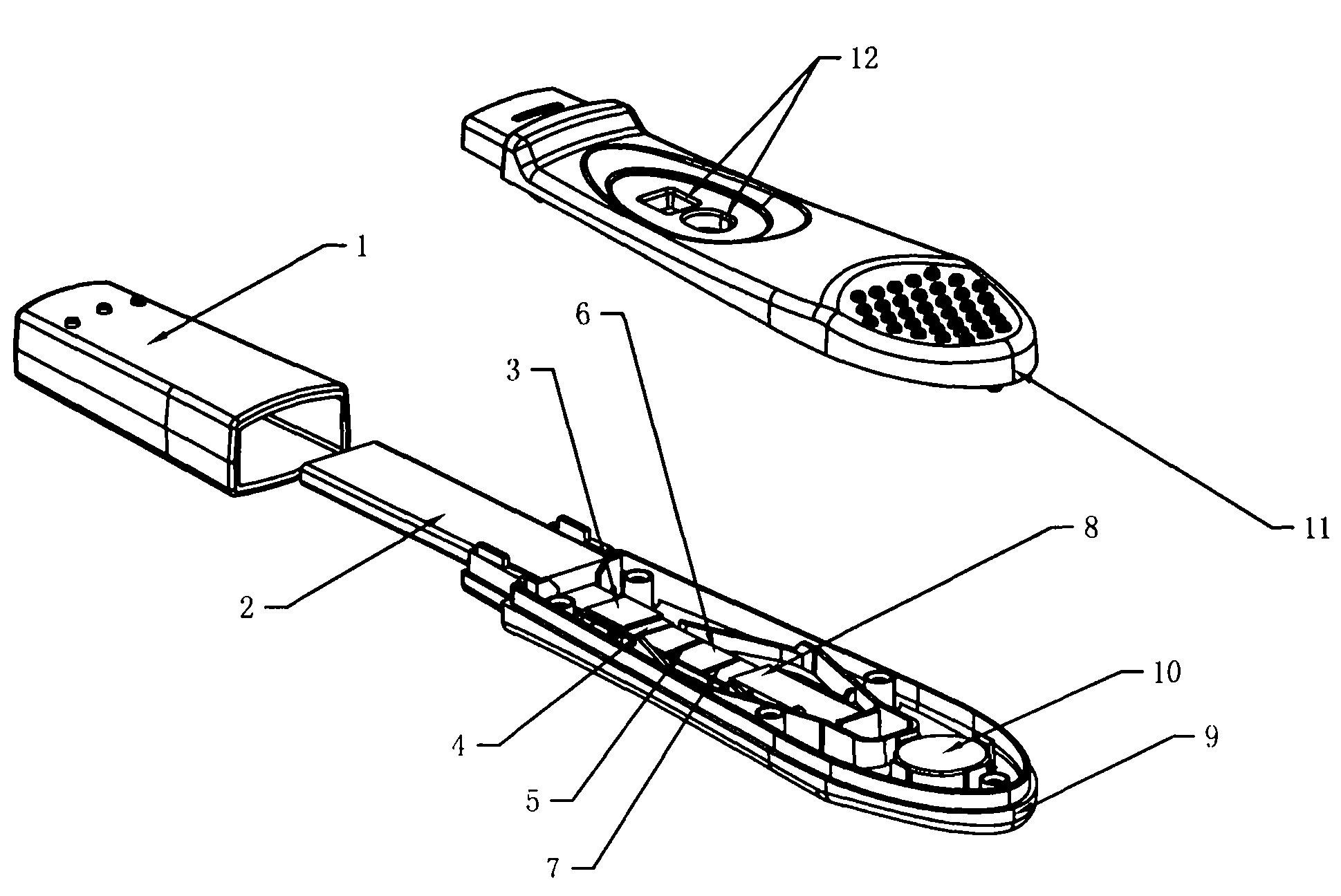
[0030] figure 1 The preparation method of the test pen shown is as follows:
[0031] 1. Preparation of colloidal gold: Trisodium citrate reduction method (HAuCl 4 , Trisodium citrate was purchased from Sigma Company) to prepare colloidal gold, and the particle size of colloidal gold was about 40nm.
[0032] 2. Preparation of Anti-IFN-γ Antibody Colloidal Gold Pads
[0033] Use colloidal gold to label anti-IFN-γ monoclonal antibody (Abcam) at a ratio of 1mg antibody / 1500D colloidal gold at pH 8.4. After 30 minutes, add an appropriate amount of BSA at pH 7.4 and concentrate by centrifugation After that, the IFN-γ antibody gold probe was obtained.
[0034] The gold probe was diluted to OD2.0 with colloidal gold film diluent (0.1M TAPS buffer, 20% sucrose, 3.75% BSA, pH 8.0) for use.
[0035] After fully immersing the glass fiber (Millipore company) in the IFN-γ collo...
Embodiment 2
[0046] Example 2, detect Mycobacterium tuberculosis by detecting IFN-γ in human blood
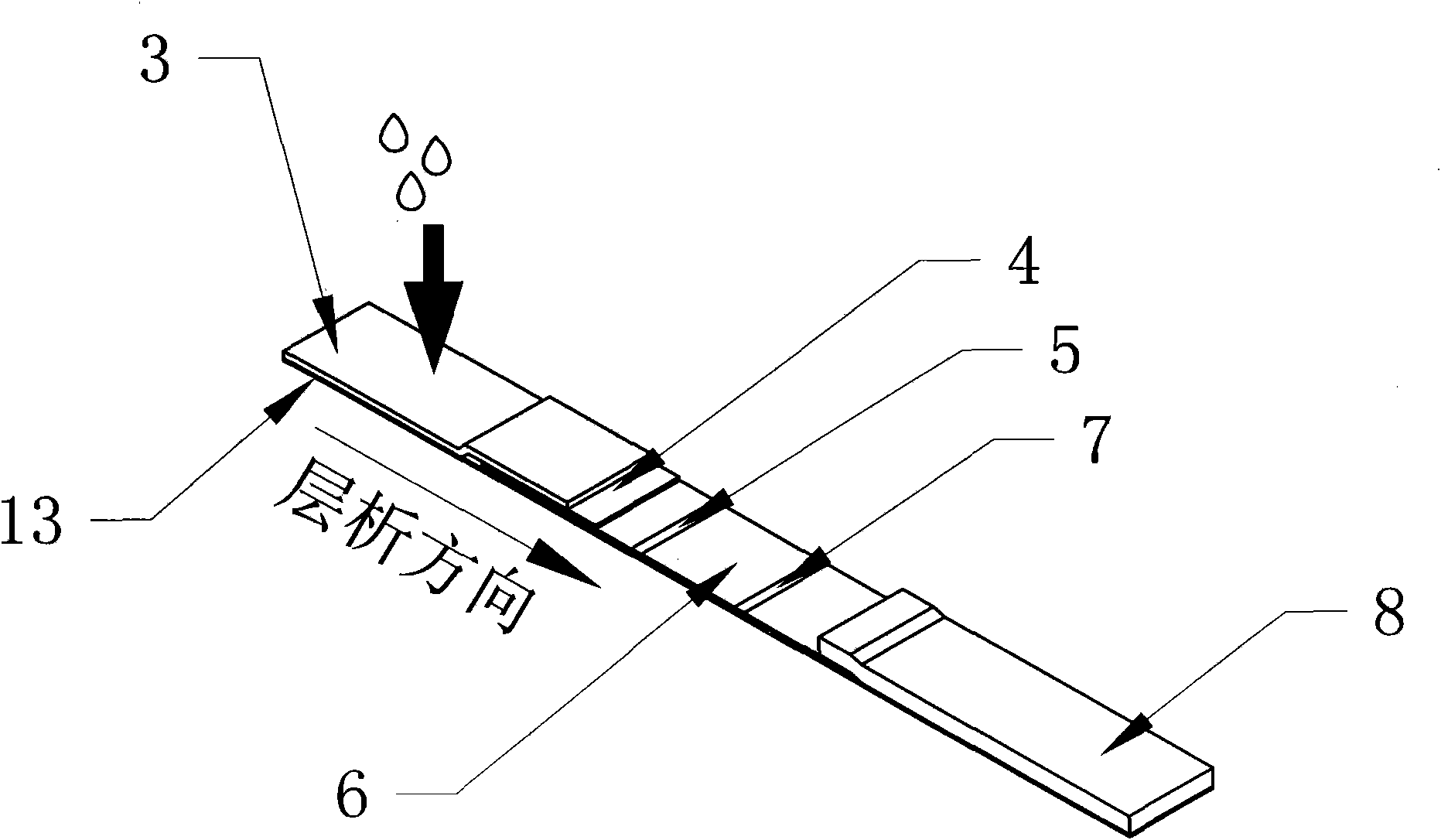
[0047] In the first step, a blood sample is collected. Take 1ml of blood from the patient and add it to a sterile test tube containing heparin (Hong Kong Advanced Technology Industries Co., Ltd.) as a negative control tube; ) in a sterile test tube as a measuring tube; then draw 1ml of blood from the patient, and add heparin and cell mitogens (such as phytohemagglutinin, Hong Kong Advanced Technology Industries Co., Ltd.) into a positive control tube. Shake the 3 tubes of blood fully respectively. Start the detection test within 12 hours. The second step is to incubate the sample. After blood samples were collected, 3 tubes were incubated at 37°C for 6-24 hours within 12 hours. The third step is sample testing. Take 100 μl sample dropwise into figure 1 The colloidal gold immunochromatographic test pen for detecting IFN-γ (included in the box) figure 2 If there is a red line at the c...
Embodiment 3
[0052] Embodiment 3, detect HIV by detecting IFN-γ in human blood
[0053] In the first step, a blood sample is collected. Take 1ml of blood from the patient and add it to a sterile test tube containing heparin (Hong Kong Advanced Technology Industries Co., Ltd.) as a negative control tube; Industrial Co., Ltd.) as a measuring tube; then draw 1ml of blood from the patient, and add heparin and mitogens (such as phytohemagglutinin, phytohemagglutinin, Hong Kong Advanced Technology Industries Co., Ltd.) as a positive control tube. Shake the 3 tubes of blood fully respectively. Start the detection test within 12 hours. The second step is to incubate the sample. After blood samples were collected, 3 tubes were incubated at 37°C for 6-24 hours within 12 hours. The third step is sample testing. Take 100 μl sample dropwise into figure 1 The test pen for detecting IFN-γ as shown (included in the box) figure 2 Show the colloidal gold immunochromatography test strip that detects ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com