Hepatitis B treating vaccine prepn and its prepn process and use
A hepatitis B and hepatitis B vaccine technology, applied in medical preparations containing active ingredients, antiviral agents, pharmaceutical formulations, etc., can solve the problem of not achieving promotion or recovery of immune regulation, inconsistent treatment dose, treatment course, and lack of hepatitis B vaccine. Standardization and other issues to achieve the effect of maintaining concentration, increasing single batch yield, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
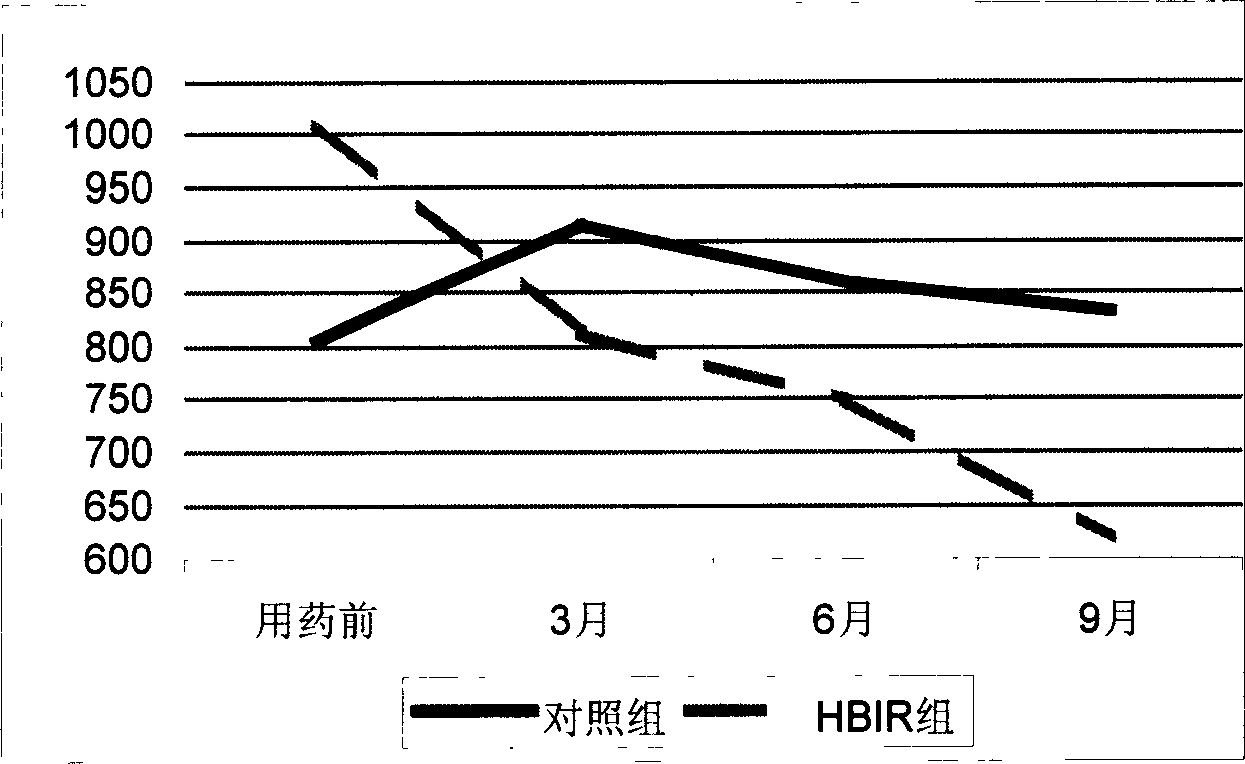
Image
Examples
Embodiment 1
[0069] This embodiment relates to hepatitis B vaccine (HBsAg) acute toxicity experimental research
[0070] 1 Materials and methods
[0071] 1.1 Instruments and medicines, diluents
[0072] Therapeutic hepatitis B vaccine 1ml each, containing HBsAg 60μg, aluminum 0.5mg, batch number: T010702, aluminum diluent containing aluminum 0.5mg / ml, Hitachi 7020 automatic biochemical analyzer, AC920 EO Hematology analyzer (Sweden), ELISA detector and reagents (HELIX, USA).
[0073] 1.2 Experimental animals
[0074] Clean-grade SD rats and SPF-grade NIH mice were provided by the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine. Rats weigh 200-240 grams, mice: 20-23 grams for females, 23-27 grams for males. Healthy cynomolgus monkeys were provided by Guangdong Shunde Institute of Experimental Animals, aged 2-3 years.
[0075] 1.3 Acute toxicity test method in mice
[0076] NIH mice were randomly divided into three groups according to sex and body wei...
Embodiment 2
[0120] This example relates to the effect of hepatitis B vaccine (HBsAg) on mouse cellular immune response
[0121] 1.1. Materials:
[0122] Recombinant (yeast) hepatitis B vaccine [Recombinant Hepatitis B Vaccine (yeast), hereinafter referred to as RHBV], each 1ml, mainly containing recombinant (yeast) HBsAg subunit 60μg, all solvents use aluminum diluent 0.5mg / ml (Shenzhen Kangtai Biological Products Co., Ltd. product). As the experimental animals, 6-8 week-old SPF Balb / c mice were used, half male and half female, weighing 16-18 g (purchased from the Department of Experimental Animals, Tongji Hospital, Huazhong University of Science and Technology). The main reagents and instruments are RPMI-1640 medium (Sigma Company), calf serum (Sijiqing Biological Company), tritium-thymidine ( 3 H-TdR, Beijing Institute of Atomic Energy), IL-2, IFN-γ detection kit (Shenzhen Jingmei Bioengineering Company), hepatitis B surface antibody detection kit (Shanghai Industry Kehua Biotechnol...
Embodiment 3
[0179] This embodiment relates to the impact of hepatitis B vaccine (HBsAg) on HBV markers in HBV gene mice
[0180] 1. Materials and Methods
[0181] 1.1 HBsAg vaccine: Provided by Shenzhen Kangtai Biological Products Co., Ltd., batch number: T010702, 1ml each, containing 60 μg of recombinant (yeast) HBsAg subunit, solvents are aluminum diluent (0.5mg / ml), batch number: A010621.
[0182] 1.2 Experimental animals: HBV transgenic mice: SPF grade, certificate of conformity: No. 2002A001, half male and half male, weighing 16-18 grams. The center builds itself, raises and breeds.
[0183] 1.3 Main detection reagents HBsAg EIA detection kit: Shanghai Industrial Kehua Biotechnology Co., Ltd., HBsAg solid-phase rapid method quantitative kit: Institute of Isotopes, China Institute of Atomic Energy, HBsAg immunohistochemical reagents: Dako company, American company HBV DNA fluorescence Quantitative PCR kit: American AmgGen company
[0184] 1.4 Grouping and administration of mice: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com