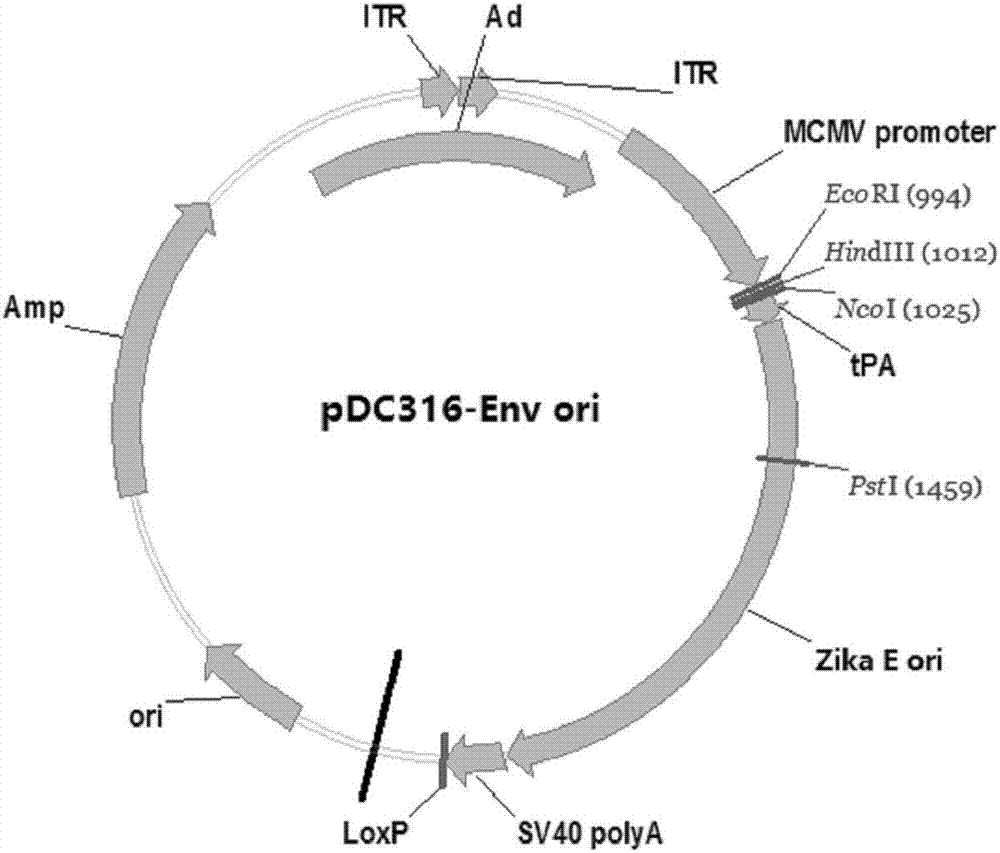
Zika virus disease vaccine taking human Ad5 replication-defective adenovirus as vector
A technology for Zika virus and replication defects, applied in the field of bioengineering, can solve the problems of inefficient vaccine prevention, unfavorable emergency immunization of large-scale population, multiple immunizations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
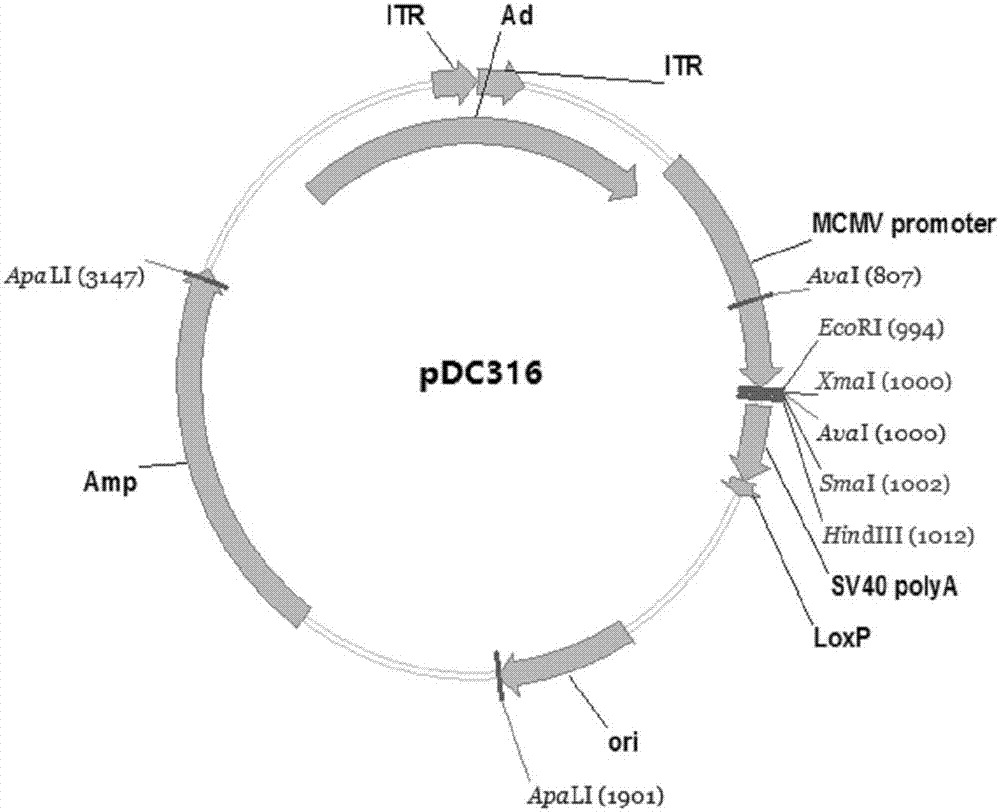
Image
Examples
Embodiment 1
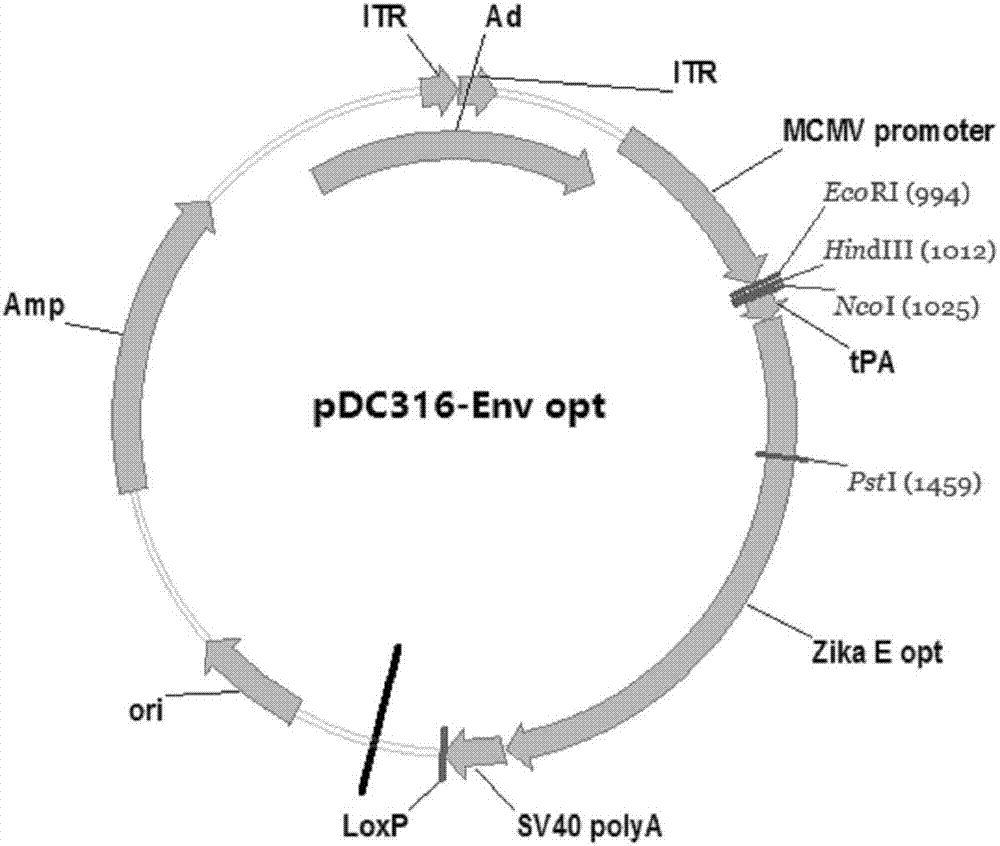
[0040] Example 1: Preparation of Zika virus disease vaccine with human replication defective adenovirus as carrier
[0041] 1. Optimization and synthesis of ZIKV prM endogenous signal peptide codon sequence, prM protein codon sequence and Env protein codon sequence.
[0042] Using the software Upgene (Gao, W., Rzewski, A., Sun, H., Robbins, P.D., & Gambotto, A. (2004). UpGene: Application of a web-based DNA codon optimization algorithm. Biotechnol Prog, 20 (2) , 443-448.doi:10.1021 / bp0300467) for the ZIKV prM endogenous signal peptide codon sequence and prM protein codon sequence of the ZIKV strain MRS_OPY_Martinique_PaRi_2015 (GeneBank Accession No KU647676) isolated during the Martinique outbreak in the Caribbean in 2015 And Env protein codon sequence is optimized to make it more suitable for expression in mammalian cells. The optimized codon sequence of the ZIKV prM endogenous signal peptide is shown in SEQ ID NO.3, and the oligonucleotide fragments are synthesized accordi...
Embodiment 2
[0100] Example 2 Immunological evaluation of Zika virus disease vaccine on mouse model.
[0101] In the mouse model, the immunization dose of the recombinant adenovirus vector ZIKV vaccine was studied, and the level of humoral immunity and cellular immunity induced by the vaccine was evaluated.
[0102] 1. Evaluation and comparison of humoral immune response induced by recombinant adenovirus vector ZIKV vaccine Ad5-Env and Ad5-Sig-prM-Env
[0103] 1.1 Comparison of vaccine immunization doses and evaluation of humoral immune response in mice
[0104] SPF grade female BALB / c mice (4-6 weeks old) were purchased from Victoria Levar. They were kept in the animal room of Fengtai Institute of Military Medical Sciences. The injection volume was 50 μl, blood was collected every week after immunization, and the prepared serum was frozen and stored at -20°C for future use. The mouse grouping, immunization dose and immunization site are shown in Table 2-4: Table 2: Ad5-Env immunization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com