Immune adjuvant and application thereof
An immune adjuvant and vaccine technology, applied in antiviral agents, aerosol delivery, medical preparations containing active ingredients, etc., can solve the problems of poor immune regulation effect, achieve good adjuvant effect, and enhance different antigenic humors Effects of immune response and mucosal immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Preparation of 1% chitosan and its derivative solution
[0052] Primary material sources:
[0053] Chitosan and chitosan acetate were purchased from HEPPE MEDICAL CHITOSAN GmbH
[0054] Chitosan quaternary ammonium salt was purchased from Nantong Lushen Biological Engineering Co., Ltd.
[0055] Methylene glycol chitosan was purchased from SIGMA
[0056] (1) Preparation of 1% chitosan quaternary ammonium salt (HTCC) solution
[0057] Accurately weighed 0.5 gram of chitosan quaternary ammonium salt is placed in a beaker with a magnetic stirrer, adds 50ml0.3% acetic acid solution, magnetic stirrer 200rpm stirs for 30 to 45 minutes, the chitosan quaternary ammonium salt after dissolving The ammonium salt solution was centrifuged at 4000rmp for 10 minutes, and finally the centrifuged supernatant was passed through a 0.45 μm filter membrane, and stored at 2-8°C for later use.
[0058] (2) Preparation of 1% methyl glycol chitosan (MGC) solution and chitosan a...
Embodiment 2
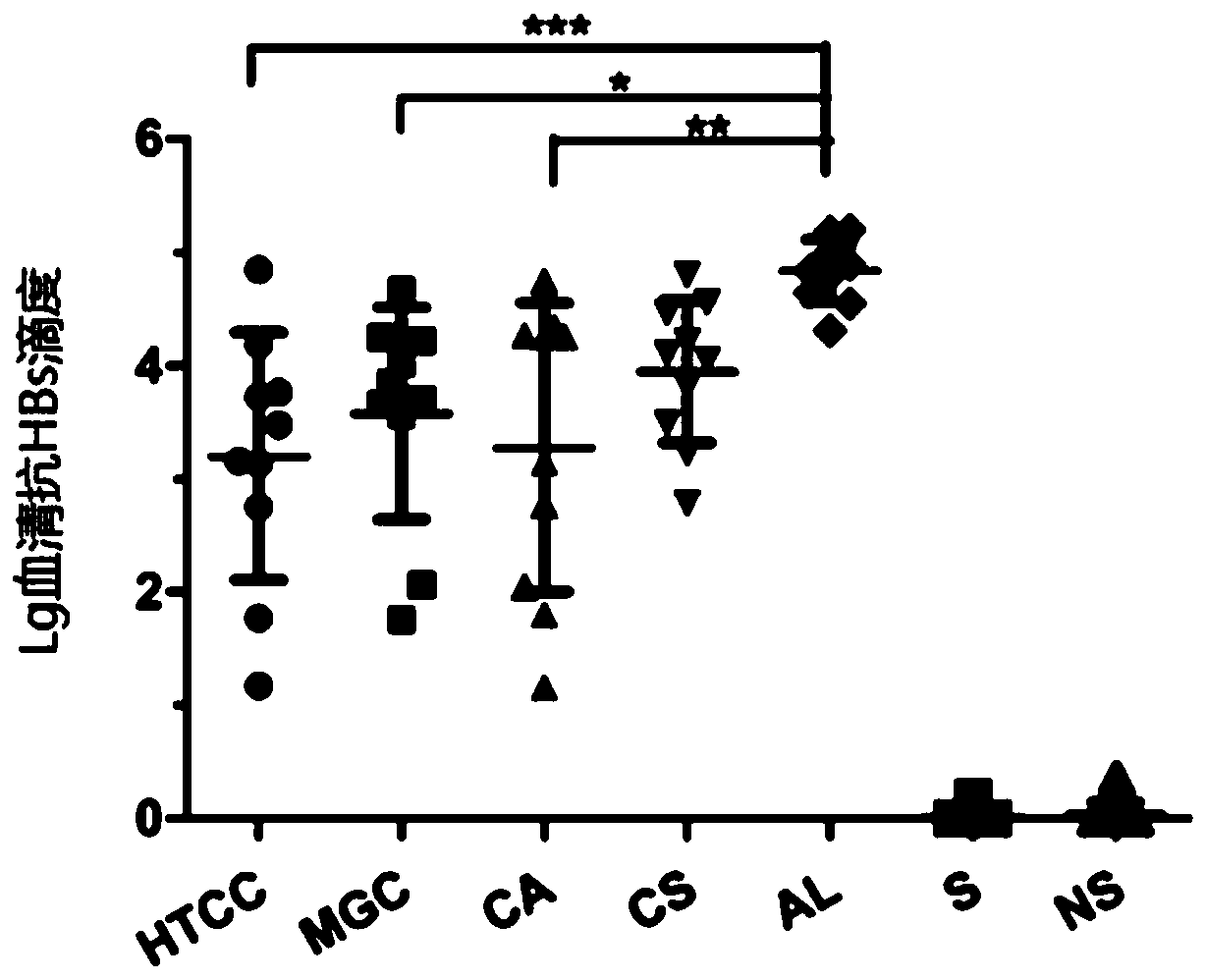
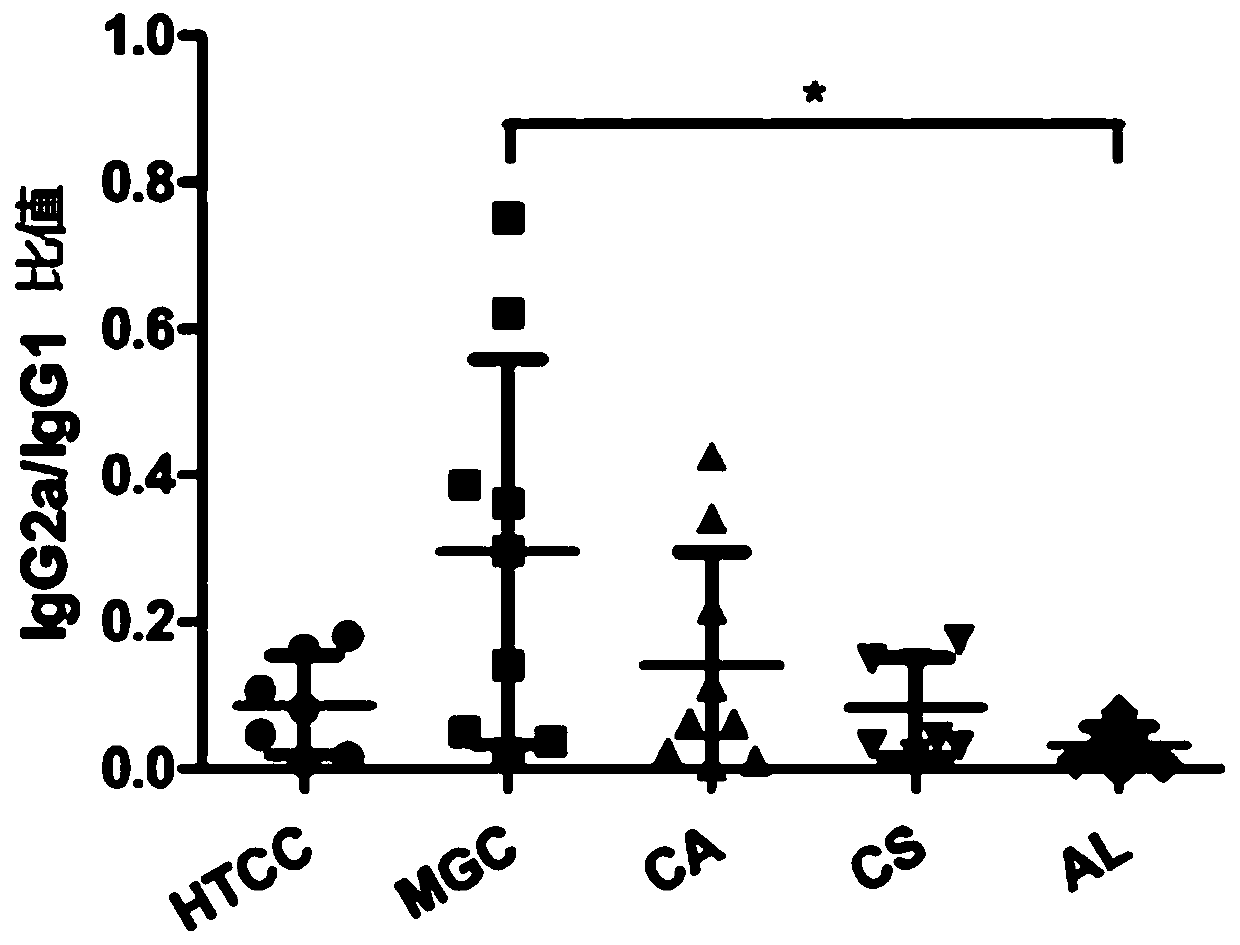
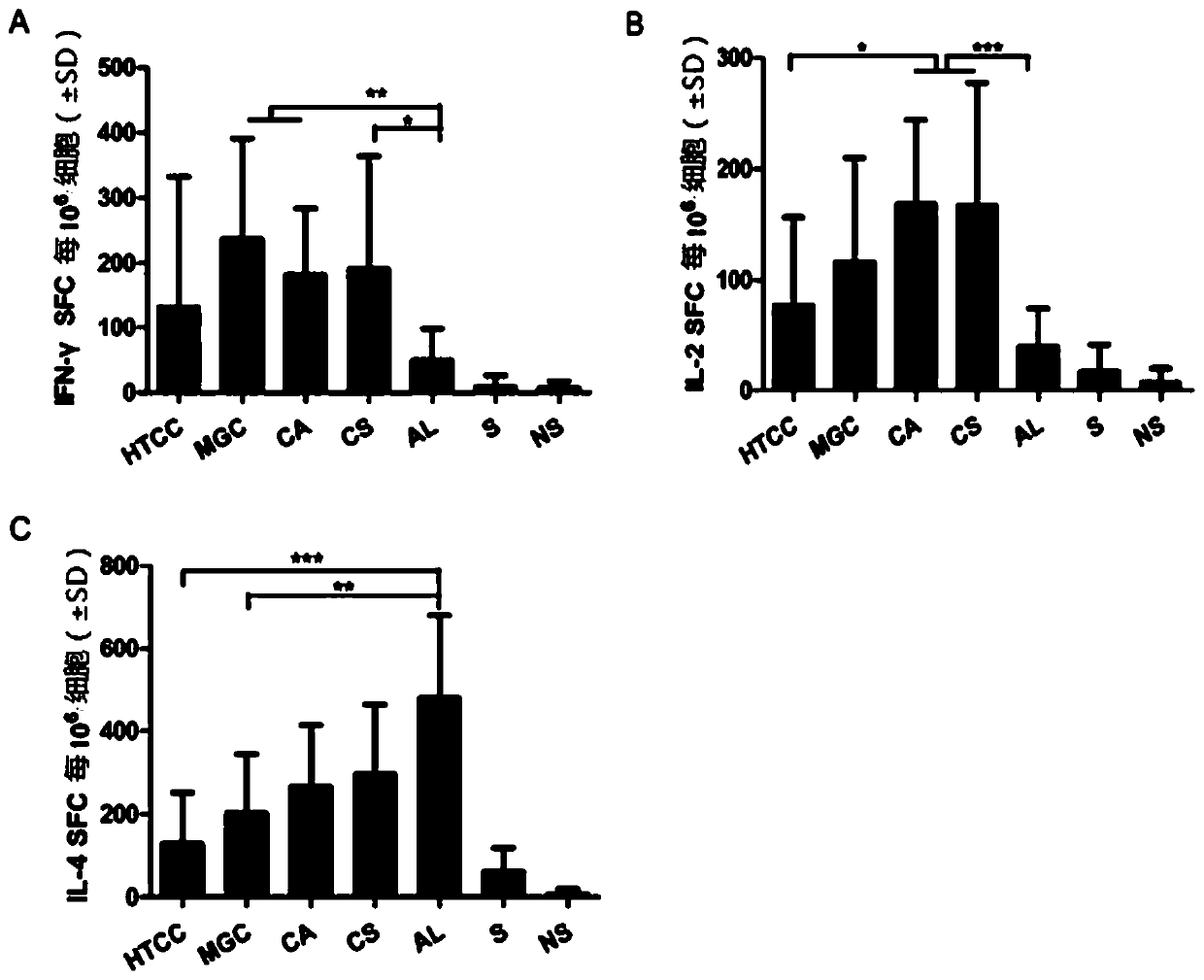
[0062] Example 2: Evaluation of the mucosal immune effect of chitosan derivatives as hepatitis B vaccine adjuvants
[0063]Chitosan and its derivatives in Example 1 are used as vaccine adjuvants, and recombinant hepatitis B subunit vaccine (Saccharomyces cerevisiae expresses HBsAg, produced by Beijing Tiantan Biological Products Co., Ltd.) is used as antigen. Take the preparation of 1ml of vaccine with chitosan or its derivatives as an example, take 100μl of chitosan or its derivatives solution, add 100μl of PBS and mix well, then add 200μl of normal saline, and finally add 550μl of HBsAg solution Mix quickly and store at 2-8°C. Used to immunize mice with intranasal drops, and detect anti-HBs in mouse serum, the specific method is as follows:
[0064] Experimental animals: BALB / c mice, 6-8 weeks old, 13 mice / group, female, purchased from Weitong Lihua Experimental Animal Technology Co., Ltd.
[0065] Drug concentration: chitosan and its derivatives prepared in Example 1 abov...
Embodiment 3
[0120] Embodiment 3: Chitosan derivative is used as the IgG of the mucous membrane immune adjuvant of influenza vaccine and hemagglutination inhibitory titer evaluation
[0121] Chitosan and derivatives thereof prepared in Example 1 were used as vaccine adjuvant, and H1N1 influenza vaccine (H1N1 influenza virus lysate, Beijing Tiantan Biological Products Co., Ltd.) was used as antigen. The two prepared vaccines according to the preparation method of hepatitis B vaccine in Example 2, immunized mice with nasal drops, and detected serum-specific IgG and hemagglutination inhibitory titers. The specific methods are as follows:
[0122] Experimental animals: BALB / c mice, 6-8 weeks old, 10 mice / group, female, purchased from Weitong Lihua Experimental Animal Technology Co., Ltd.
[0123] Drug concentration: Chitosan and its derivatives prepared in Example 1 above: 10 mg / ml; the final concentration of HA in the H1N1 lysate is 562 μg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com