Application of chitosan oligosaccharide in preparing vaccine adjuvant and vaccine composition
A technology of vaccine composition and vaccine adjuvant, which is applied in the field of preparation of vaccine adjuvant and vaccine composition and vaccine preparation containing the vaccine adjuvant, can solve the problems of high price and application limitation, and achieve reasonable cost and controllable quality , easy absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
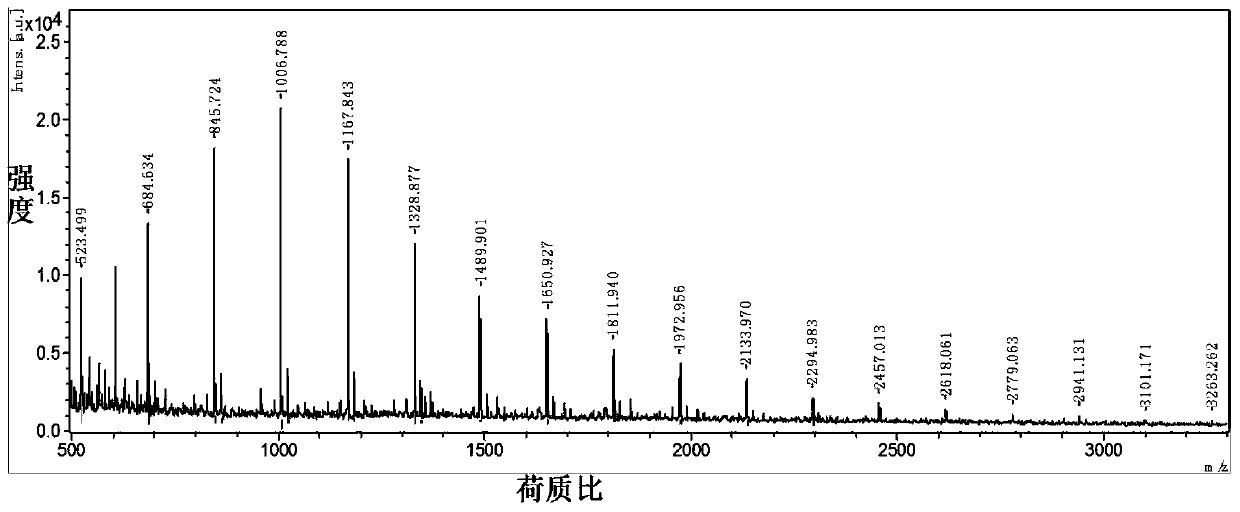
[0033] Embodiment 1: preparation and physical and chemical properties of chitosan oligosaccharide
[0034] 1. Preparation of chitosan oligosaccharide:
[0035] Add deionized water into the batching tank, dissolve chitosan in 1% acetic acid solution, stir and heat up to 38±2.0°C. Add 5% (to the substrate) enzyme preparation, stir, and keep the temperature at 38°C. After the viscosity of the enzymolysis solution drops to 60%, start ultrafiltration (cut molecular weight at 6000). The part smaller than the cut-off molecular weight permeates the membrane, and the part larger than the cut-off molecular weight returns to the reactor to continue to degrade. The ultrafiltration permeate is passed through a nanomembrane filter (cutting molecular weight 300), and the permeated part is water, acetic acid and small molecule sugar (the permeated part can be recycled for preparing chitosan solution), and the non-permeated part is Concentrate into chitosan oligosaccharide solution and spr...
Embodiment 2
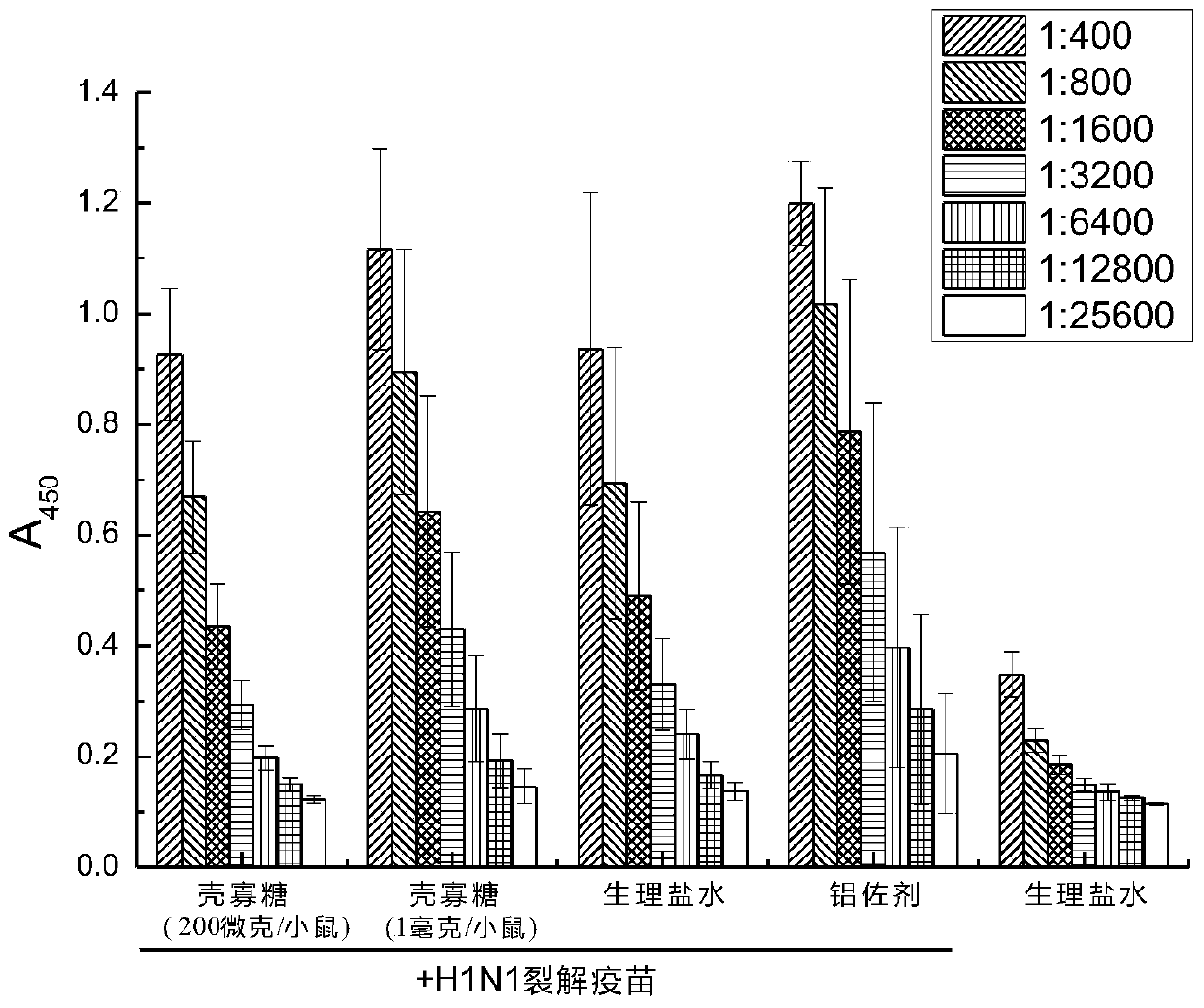
[0045] Embodiment 2: the adjuvant activity determination of chitosan oligosaccharide to influenza vaccine
[0046] The chitosan oligosaccharide in Example 1 was used as a vaccine adjuvant, and the H1N1 influenza vaccine (H1N1 influenza virus lysate, 30 μg / ml) was used as an antigen, and the two were injected intramuscularly to immunize mice, and the antibody titer was determined. The specific method is as follows:
[0047] Experimental animals: BALb / c mice, 6-8 weeks old, 5 mice / group, female.
[0048] Drug concentration: Chitooligosaccharide (COS) prepared in Example 1 above: 4 mg / ml and 20 mg / ml; H1N1 influenza virus lysate: 30 μg / ml; aluminum adjuvant (Al): 4 mg / ml.
[0049] Control solvent: physiological saline (Saline).
[0050] Drug concentration after mixing: chitosan oligosaccharide: 2 mg / ml and 10 mg / ml; H1N1 influenza virus lysate: 15 μg / ml; aluminum adjuvant (Al): 2 mg / ml.
[0051] Dosage: Oligochitosan: 200 μg / mouse (mouse) and 1 mg / mouse; H1N1: 1.5 μg / mouse; ...
Embodiment 3
[0066] Embodiment 3: the adjuvant activity of chitosan oligosaccharide to hepatitis B vaccine
[0067] The chitosan oligosaccharide in Example 1 was used as a vaccine adjuvant, and the recombinant hepatitis B subunit vaccine (HBsAg expressed by Hansenula yeast, produced by Dalian Hanxin Biomedical Co., Ltd.) was used as an antigen, and the two were used to immunize mice by intramuscular injection , Determination of antibody titers. Concrete experiment is with embodiment 2.
[0068] Dosage: Oligochitosan: 200 μg / mouse (mouse) and 50 μg / mouse; HBsAg: 2 μg / mouse; Aluminum adjuvant (Al): 200 μg / mouse.
[0069] Experimental grouping: (1) normal saline group (Saline); (2) aluminum adjuvant group (Al+HBsAg); (3) hepatitis B subunit group (Saline+HBsAg); (4) COS low-dose group: COS (200μg / mouse)+HBsAg; (5) COS high dose group: COS (1mg / mouse)+HBsAg.
[0070] Equal volumes were mixed before injection, 100 μl / mouse, and intramuscularly injected into the right hind limb.
[0071] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com