Delivery system and application of chitosan mucosa compromising mucosal adjuvant
A technology of chitosan and mucosal adjuvant, applied in the fields of biochemical drugs and chemistry, can solve the problems that antigens cannot stay, cannot induce mucosal immune responses, etc., and achieve the effect of enhancing induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Hela cell culture and CVB3 virus passage:
[0067] The human cervical cancer cell line Hela cell of the present invention is cultured according to conventional methods, with the RPMI-1640 medium containing 10% NBS, 2mM L-glutamine, 100U / ml penicillin and kanamycin sulfate at 37 ℃, 6% CO 2 cultured under the same conditions and passaged every other day. Infect approximately 5×10 6 For Hela cells, 80% of the cells were lysed by replicating virus after 40 hours of culture, and the liquid and cell fragments were centrifuged at 3000 rpm / min for 20 minutes, and the obtained supernatant was fresh CVB3 suspension.
Embodiment 2
[0068] LD of embodiment 2 CVB3 virus 50 (LD50) titration:
[0069] The 48-hr BALB / c suckling mice were taken out and divided into 8 groups, with 5 mice in each group, and the freshly prepared CVB3 suspension was sequentially diluted to 10 with NS in 10-fold serial dilution method. -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 , respectively take 40μl and inject 8 groups of suckling mice intraperitoneally, observe the survival of suckling mice every day, and calculate the LD of this batch of CVB3 virus according to the routine method of virology 50 potency.
[0070] Distance ratio = (>50% death percentage - 50) / (>50% death percentage - <50% death percentage)
[0071] LD 50 => 50% of the logarithm of the mortality dilution (Log) + distance ratio
[0072] LD of CVB3 in the same batch in this test 50 Valence is 10 -5.5 / 100μl
Embodiment 3
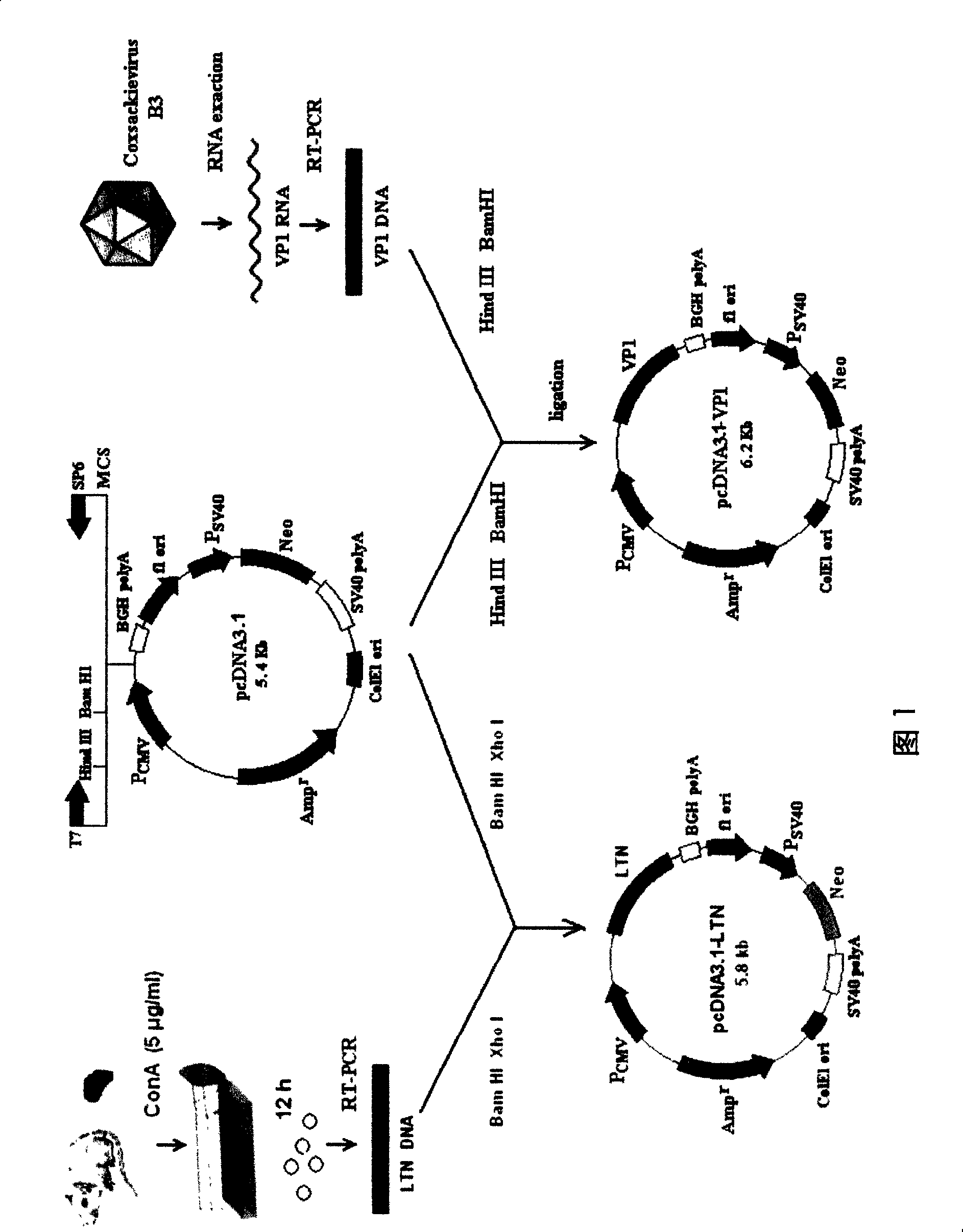
[0073] Example 3 Construction, in vitro expression and functional identification of target antigen gene and mucosal adjuvant gene plasmid DNA
[0074](1) (as shown in Figure 1) Construction of target antigen plasmid and mucosal adjuvant encoding plasmid: taking CVB3 structural protein VP1 as an example of target antigen and chemokine Ltn as an example of mucosal adjuvant, a pcDNA3. 1 is the pcDNA3.1-VP1 and pcDNA3.1-Ltn plasmids of the vector (or pVAX vector). Among them, the VP1 cDNA sequence is based on the literature (Klump WM, et al. J Virol, 1990, 64 (4): 1573-1583); the gene sequence of LTN is derived from NCBI GeneBank (NM-008510) as follows: agcccagcaa gacctcagcc atgagacttc tcctcctgac tttcctggga gtctgctgcctcaccccatg ggttgtggaa ggtgtgggga ctgaagtcct agaagagagt agctgtgtga acttacaaacccagcggctg ccagttcaaa aaatcaagac ctatatcatc tgggaggggg ccatgagagc tgtaatttttgtcaccaaac gaggactaaa aatttgtgct gatccagaag ccaaatgggt gaaagcagcg atcaagactgtggatggcag ggccagtacc agaaagaaca tggctga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com