Curdlan sulfate/6-O-quaternized chitosan nanoparticles and application thereof in mucosal vaccines
A technology of quaternized chitosan and mucosal vaccines, which is applied to medical preparations containing active ingredients, antibody medical ingredients, drug combinations, etc., can solve the problems of difficult passage of macromolecular protein antigens and weak enhancement effects, and achieve Improve the level of cellular immunity and humoral immunity, stimulate proliferation and differentiation, and promote the effect of activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Concrete, the preparation method of described nanoparticle, comprises the following steps:
[0052] 1) Put sulfated codran polysaccharide (CS) in deionized water, dissolve, filter and dilute to obtain solution A;
[0053] 2) Place 6-O-quaternized chitosan (O-HTCC) in deionized water, dissolve, filter, and dilute to obtain solution B;
[0054] 3) Slowly add solution B to solution A under stirring, and continue to stir for a period of time to obtain sulfated codranan / 6-O-quaternized chitosan nanoparticle solution;
[0055] Preferably, the filtration in step 1) and step 2) is treated with a filter membrane with a diameter of 0.22 μm;
[0056] Preferably, the molecular weight of sulfated candella polysaccharide is 103kD, the molecular weight of 6-O-quaternized chitosan is 100kD~200kD (most preferably 100kD), and the degree of quaternization is not less than 30%;
[0057] Preferably, the concentration ratio of the solution A to the solution B is 1:4-6 (most preferably 1:5)...
Embodiment 1
[0077] The preparation of embodiment 1 OVA / CS / O-HTCC nanoparticles
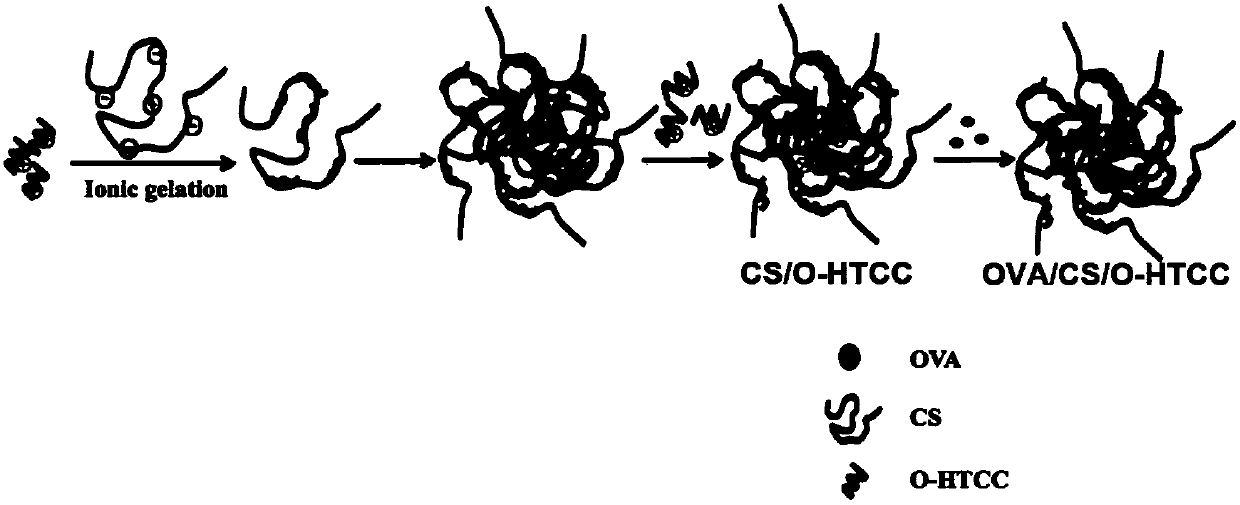
[0078] The preparation process is as figure 1 As shown, 10 mg of sulfated candlan was dissolved in 10 mL of deionized water to prepare a 1 mg / mL sulfated candlan mother liquor. After filtering with a filter membrane with a diameter of 0.22 μm, take 100 μL of the mother liquor of sulfated ketanan and dilute it with 900 μL of deionized water to obtain a 100 μg / mL sulfated ketanan solution; take 10 mg of 6-O-quaternized chitosan and dissolve it in In 10 mL of deionized water, a 1 mg / mL 6-O-quaternized chitosan mother solution was prepared. After filtering with a filter membrane with a diameter of 0.22 μm, 500 μL of the 1 mg / mL 6-O-quaternized chitosan mother solution was diluted with 500 μL of deionized water to obtain a 500 μg / mL 6-O-quaternized chitosan solution.
[0079]Take 500 mL of 100 μg / mL sulfated kurtlan solution and add 500 mL of 500 μg / mL 6-O-quaternized chitosan prepared in the previous stage drop...
Embodiment 2
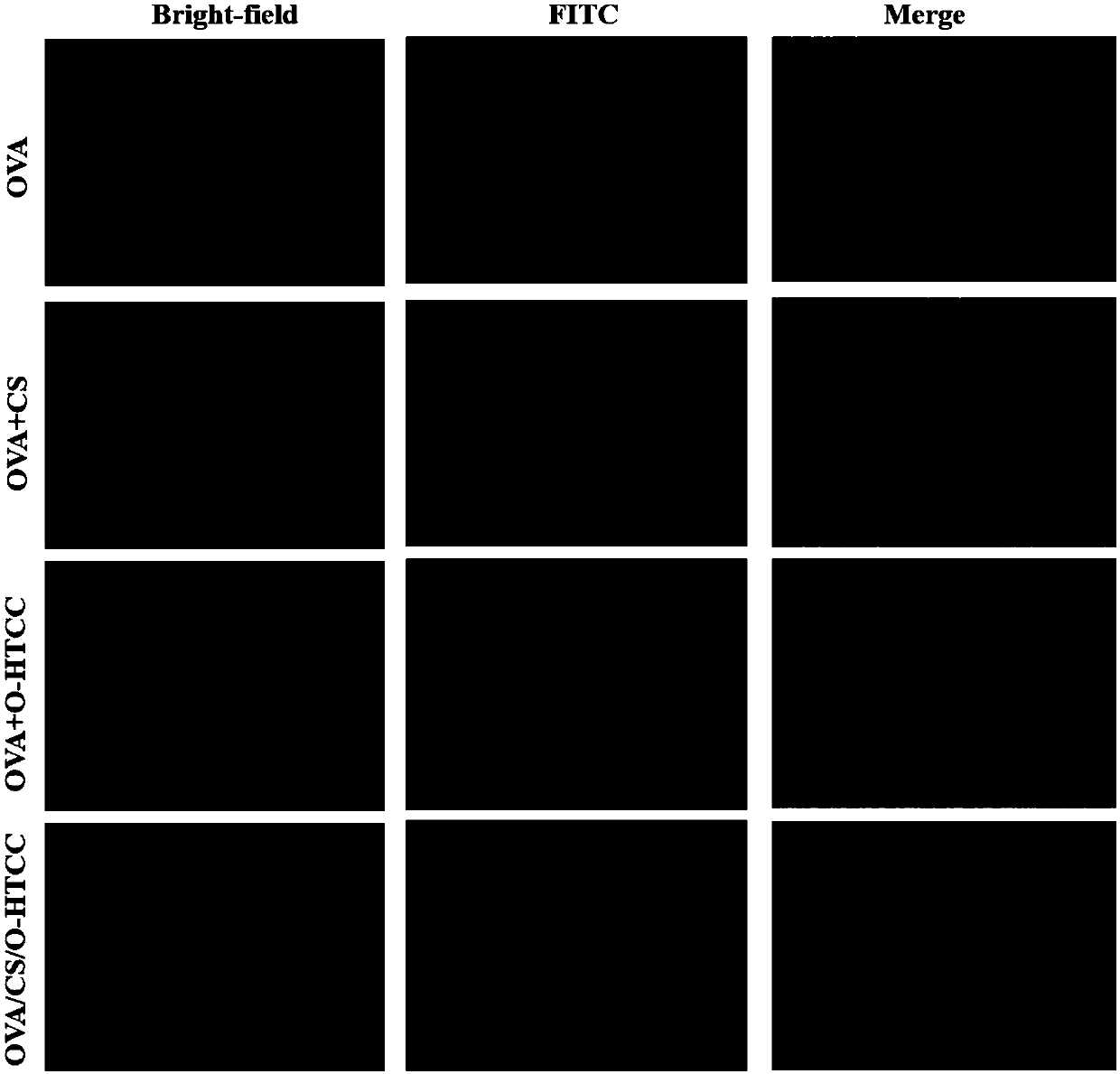
[0083] Example 2 Detection of uptake capacity of FITC-labeled OVA / CS / O-HTCC nanoparticles
[0084] To investigate the ability of OVA / CS / O-HTCC nanoparticles to be taken up by epithelial cells, the Calu-3 cell line with nasal epithelial cell characteristics was selected as the experimental model, and the specific implementation steps were as follows:
[0085] 1 mL of 1 mg / mL FITC solution was added dropwise to 1 mL of OVA solution (10 mg / mL), stirred overnight at 4°C, and 7 mg of ammonium chloride was added to terminate the reaction. The reaction solution was dialyzed in the dark for 48 hours using a dialysis bag with a molecular weight cut-off of 1 kD, and then freeze-dried to obtain FITC-OVA.
[0086] Take Calu-3 cells in the logarithmic growth phase, and use 1×10 5 Inoculate into a culture dish at a density of one / dish, culture in a cell incubator for 4 days, discard the medium, add FITC-OVA, a mixture of FITC-OVA and CS, a mixture of FITC-OVA and O-HTCC or FITC -OVA / CS / O-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com