Drug for resisting Coxsackie virus and preparing method and application thereof
A coxsackie virus and drug technology, applied in the direction of antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., to achieve the effect of no toxic side effects, simple preparation method, and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Auranthus nudiflora extract (Aurora nudiflora dry extract):
[0036] The above-mentioned extract of Auranthus nudica is preferably prepared by the following method: take Auranthus nudica, cut into 1-2cm slices, add 10 times the amount of 90% ethanol, reflux extraction twice, the first time for 2 hours, the second Filtrate for 1 hour, recover ethanol from the filtrate and concentrate to a thick paste with a relative density of 1.30-1.33 (70-80°C), dry it under reduced pressure at 70-80°C, and grind it into a fine powder for later use; add 8 times the dregs respectively, 6 times the amount of water, decoct twice for 1 hour each time, filter, combine the filtrates, concentrate to a clear paste with a relative density of 1.19-1.21 (50-60°C), spray dry, combine with the above ethanol extract, and mix well , crushed and passed through an 80-mesh sieve for later use.
[0037] Preparation of casuarina extract (casuarina dry extract):
[0038] The casuarina extr...
Embodiment 2
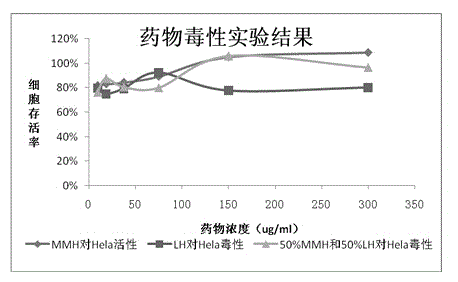
[0040] drug toxicity test
[0041] Resuscitate the cells to be tested and pave a 96-well plate with a cell density of 1*10 5 pieces / ml. After the cells grow into a monolayer, the drug to be tested is diluted to 8 concentrations (6-7 concentrations are also acceptable, that is, 10 6 -10 8), added to a 96-well plate, 100 μl per well, 6 replicate wells were set up, and a normal cell control was set at the same time. Observe under the microscope every day, and determine the maximum non-toxic concentration of the drug according to the cell morphology (compared with the normal cell group). The general state of cytotoxicity is that the cells become round and fall off. At the end of the culture (after 48 or 72 hours), 10ul / well of MTT solution (thiazolan solution) was added, and the culture was continued for 4h. Carefully aspirate MTT, add DMSO (dimethyl sulfoxide) 100ul / well, shake and incubate for 15min (be careful to avoid light during this process), after the microplate reade...
Embodiment 3
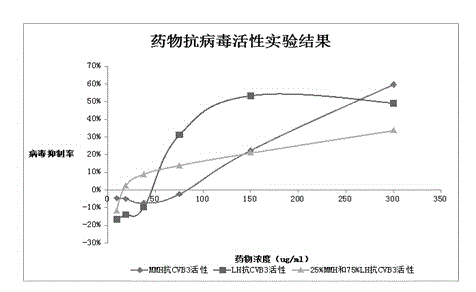
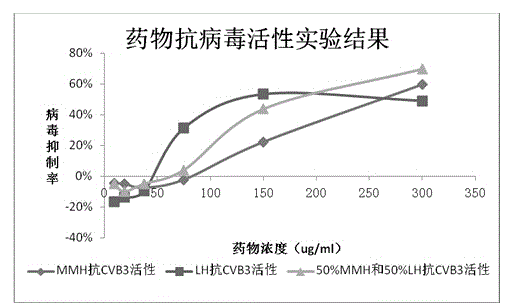
[0044] Antiviral experiment of anti-coxsackie virus drugs
[0045] After the Hela cells grow to the logarithmic phase, they are infected with the virus, and then drug solutions of different concentrations are added (doubling dilution from the maximum non-toxic concentration), and cultured in an incubator. The experiment was divided into five treatment groups:
[0046]
[0047] As described in the present invention, in above experiment, the configuration method of drug solution is as follows:
[0048] 1) Treatment groups 1 and 2
[0049] Take 300ug / mL MMH as an example: take 300ug of MMH dry extract powder (prepared in Example 1) and dissolve it in 1mL of pure water to obtain a 300ug / mL MMH solution. Other concentration 0-300 (ug / mL) group solutions are obtained by diluting the corresponding multiples with this high concentration group solution with water.
[0050] 2) Treatment group 3-5
[0051] Take 25% MMH:75% LH (1:3) as an example: its high concentration group (300u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com