A16 type strain of Coxsackie virus and application of the strain
A technology of coxsackie virus and virus strain, applied in the direction of antiviral agent, virus/phage, antiviral immunoglobulin, etc., to achieve the effect of small immune dose, stable titer and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Isolation, cultivation and identification of embodiment 1 CA16 virus strain
[0019] (1) Virus isolation and culture
[0020] The stool samples of patients with positive CA16 virus PCR results were collected from the HFMD epidemic area in Zhejiang Province in 2010 (the cumulative number of HFMD cases reached 111,783). After treatment, they were inoculated onto African green monkey kidney passage cells (Vero), and cultured for three generations for virus isolation. , after the CA16 virus was obtained, the CA16 virus strain was obtained by the plaque method.
[0021] (2) Virus identification
[0022] 1. Sequence analysis results of VP1 conserved region
[0023] The VP1 conserved region sequence of the strain was analyzed, and compared with the sequence of the reference strain (human Coxsackievirus A16 strain shzh00-1, complete genome sequence GenBank: AY790926.1), the nucleotide homology was 94.8 %above.
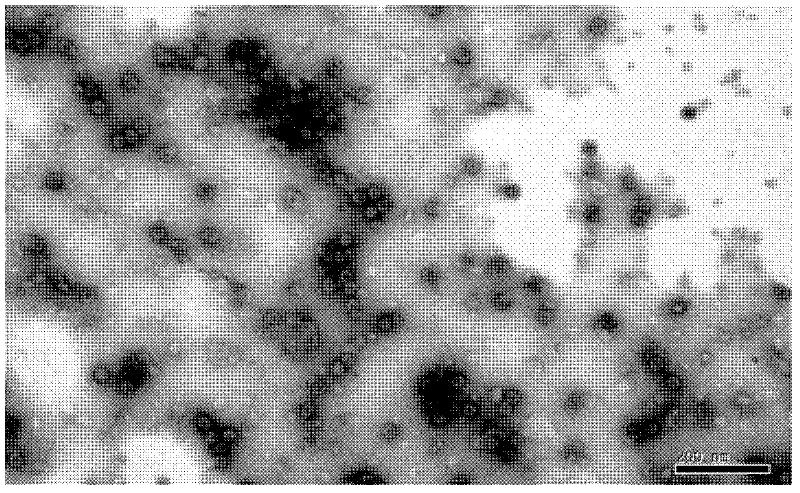
[0024] 2. Electron microscope inspection results
[0025] The...
Embodiment 2
[0034] The preparation of embodiment 2 CA16 vaccine
[0035] After the CA16 virus strain infects Vero cells, cultures, harvests the virus liquid, inactivates and purifies, a vaccine stock solution is obtained for further preparation of the CA16 vaccine.
[0036] (1) Establish cell master seed and working seed bank (Vero cell)
[0037] Resuscitate the seeds of 120-generation African green monkey kidney cells (Vero cells) from ATCC in the United States. The specific operation is: take out the cell cryopreservation tube from liquid nitrogen, place it in sterile water at 39-40°C, and thaw the cells within one minute , aseptically suck out the suspension, centrifuge at 1000rpm for 3min, discard the supernatant, add MEM cell growth medium containing 10% calf serum, mix gently by blowing, inoculate the mixed cell suspension in 25cm 2 in a cell culture flask at 37°C, 5% CO 2 Cultivate in an incubator, change the medium after it adheres to the wall, and then place it at 37°C, 5% CO ...
Embodiment 3
[0050] Example 3 CA16 strain immunogenicity test
[0051] Purify the stock solution of the CA16 strain vaccine prepared in Example 2, and after inactivation, immunize mice, New Zealand rabbits, and sheep to obtain better protective effects.
[0052] Stock solution preparation method is described with embodiment 2.
[0053] Among them, the immunogenicity test on sheep is as follows:
[0054] After absorbing the vaccine stock solution and the aluminum hydroxide adjuvant in equal proportions, the sheep were immunized on the 0th day, 7th day, 14th day, and 21st day, 2ml / head / time, and the health status, Behavioral changes, etc., are documented in detail. Animals should be observed for half an hour on the day of immunization. Animals were observed twice daily for mortality. Blood was collected on day 0, day 7, day 14, day 21 and day 28. A small amount of blood was collected venously, centrifuged at 3000rpm for 10min, and the serum was separated. Determination of anti-CA16 neu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com