Novel coronavirus detection test strip as well as preparation method and application thereof
A coronavirus and detection test strip technology, which is applied in the field of novel coronavirus detection test strips and its preparation, can solve the problems of inability to detect the incubation period and early infection patients, and achieve improved sensitivity and specificity, improved accuracy, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 The composition of the novel coronavirus detection test strip
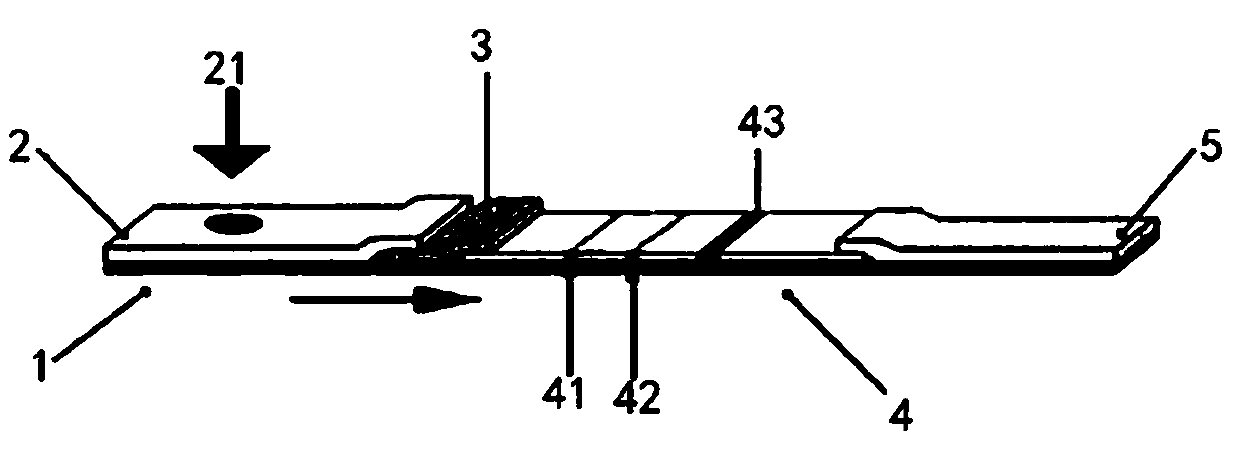
[0052] figure 2 Structural schematic diagram of the novel coronavirus detection test strip provided for the present invention; as figure 2 As shown, the new coronavirus detection test strip includes a PVC base plate 1, and a sample pad 2, a binding pad 3, an analysis membrane 4, and a water-absorbing filter paper 5 are sequentially laid on the PVC base plate 1 along the chromatography direction; the sample pad 2 is partially overlapped on the On the binding pad 3 , the binding pad 3 partially overlaps one side of the analysis membrane 4 , and the absorbent filter paper 5 partially overlaps the other side of the analysis membrane 4 , and the sample pad 2 is provided with a sampling hole 21 .
[0053] Colloidal gold-labeled 2019-nCoV recombinant antigen and colloidal gold-labeled mouse anti-human HCG monoclonal antibody are coated on the binding pad 3; the 2019-nCoV recombinant antigen carries H...
Embodiment 2
[0064] Example 2 The method of using the new coronavirus detection test strip
[0065] Step a. Return the new coronavirus detection test strip, sample diluent and sample to 18-30°C; the sample diluent is phosphate buffered saline, and the sample is serum, plasma or whole blood.
[0066] Step b. Take 10µL serum, plasma or 20µL whole blood sample and add it directly into the sample well;
[0067] Step c, add 100 µL sample diluent to the sample well;
[0068] Step d, interpret the results within 15-20 minutes, and the test results will be invalid after 20 minutes.
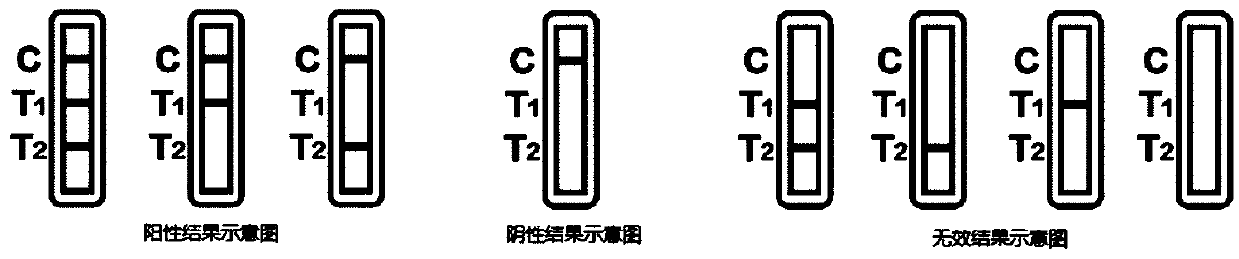
[0069] The interpretation of the test results is as follows:
[0070] 1. Positive T1 test line: If the detection window T1 test line and quality control line develop color, it indicates that the sample has detected new coronavirus IgM antibody and / or new coronavirus IgA antibody, which may be in early infection or current infection, and can be combined with Clinical symptoms are finally confirmed to improve the acc...
Embodiment 3
[0075] Example 3 Verification of novel coronavirus detection test strips
[0076] 1. Test strip performance evaluation test
[0077] Serum samples from 30 patients with novel coronavirus pneumonia (COVID-19) were clinically evaluated, and the novel coronavirus (2019-nCoV) IgA, IgM and IgG antibodies were detected by using the novel coronavirus detection test strips of the present invention respectively, and the results in Table 1 below were obtained :
[0078]
[0079] It can be seen from Table 1 that the positive results of IgA and IgM were not completely coincident. There were 15 positive cases of IgA and IgM, 11 cases of IgA antibody positive, 12 cases of IgM antibody positive, and the number of IgA antibody positive and IgM antibody negative. 3 cases, 4 cases were positive for IgM antibody and negative for IgA antibody, 1 case was positive for IgA antibody and negative for IgM antibody and IgG antibody.
[0080] 2019-nCoV IgA antibody detection can increase the detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com