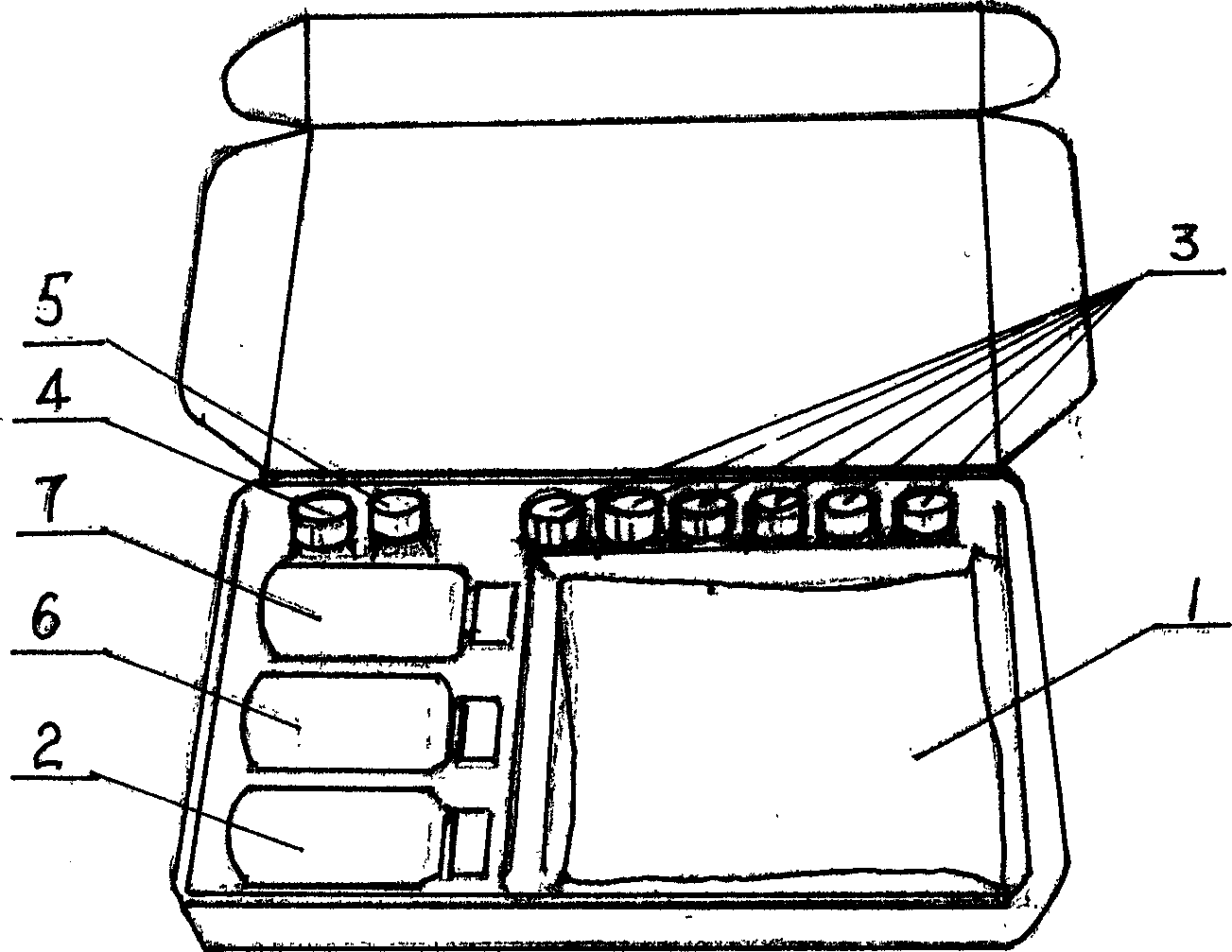
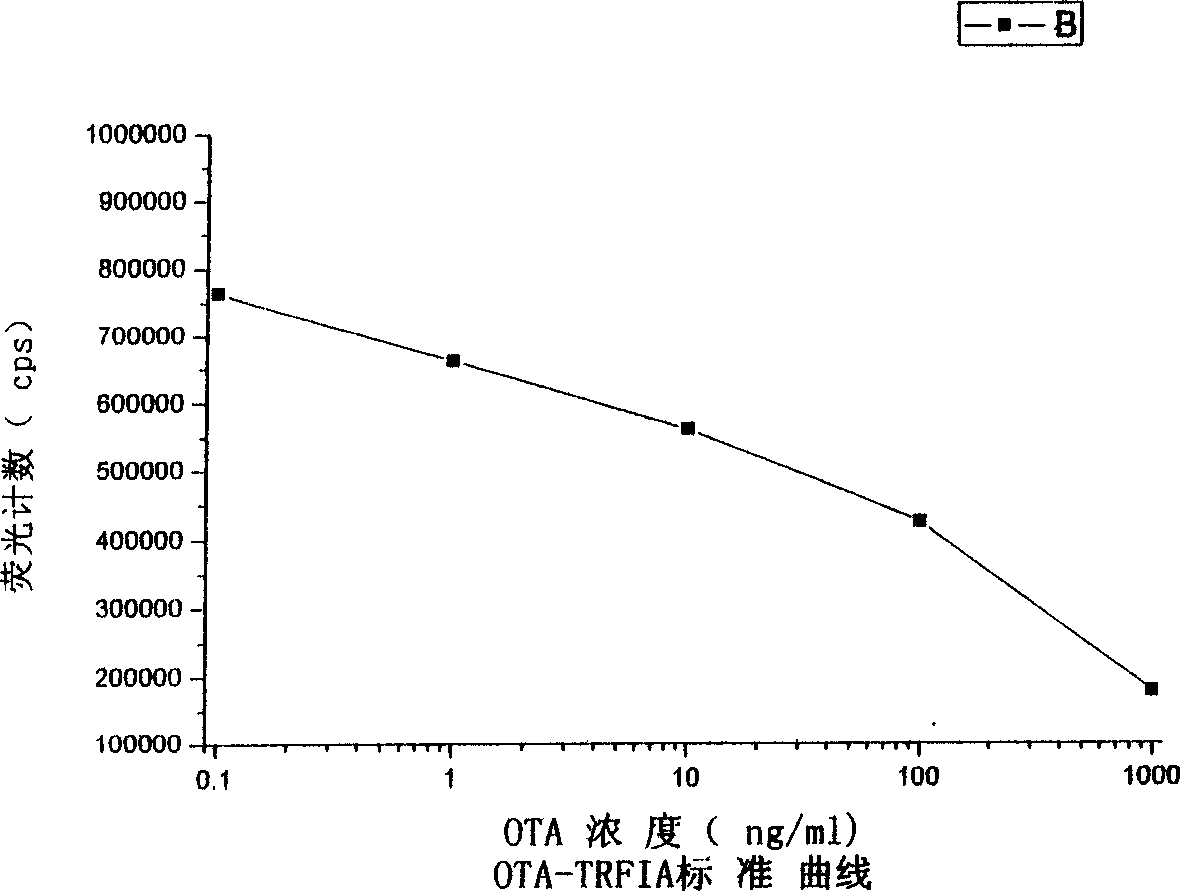
Reagent box and detection for ochracin A
An ochratoxin and kit technology, which is applied in the field of time-resolved fluorescence immunoassay, can solve the problems of unpractical application, unsatisfactory sensitivity and reagent stability, etc., and achieves the effects of simple structure, high sensitivity and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 prepares kit and detects corn sample
[0015] OTA is a typical hapten, so it has reactogenicity in the immune reaction, but it needs to be combined with macromolecular substances to be immunogenic. The carboxyl group in OTA is an active group that can combine with the amino group of the protein. Therefore, the carbodiimide method can be used to combine OTA with the macromolecular protein KLH, and use the synthetic OTA-KLH as an artificially synthesized immune antigen for animal immunization.
[0016] Preparation of OTA-KLH antigen:
[0017] 1. Dissolve 1-2mg OTA with 1ml dimethylformamide (DMF);
[0018] 2. Use 1ml of 0.13mol / L NaHCO 3 Dissolve 1-2mg KLH carrier protein in coupling buffer;
[0019] 3. Add 0.4-0.8mg OTA solution to KLH solution;
[0020] 4. Dissolve 2-4mg carbodiimide (EDC) in 1mL double-distilled water, and add 50-100μL to the above mixture, and let it work at room temperature for 2 hours (protect from light);
[0021] 5. After centrif...
Embodiment 2
[0060] Embodiment 2 prepares kit
[0061] Preparation of OTA-KLH antigen: same as Example 1.
[0062] Preparation of polyclonal ochratoxin A antibody: same as Example 1.
[0063] Eu 3+ - Preparation of goat anti-rabbit antibody:
[0064] Take 1ml of 5g / L goat anti-rabbit antibody dissolved in 50mmol / L PBS pH7.0, change the buffer condition through PD-10 column, and the eluent is 50mmol / L NaCl containing 0.155mol / L NaCl 2 CO 3 -NaHCO 3 pH9.0 buffer. The protein peaks were collected and quantified by UV absorption analysis (1.46A 280 -0.74A 260 ), dilute the goat anti-rabbit antibody to 2g / L with the eluent above. Take 500μl and add Eu containing 0.2mg 3+ -N 2 -[p-isocyanate-benzyl]-diethylenetriaminetetraacetic acid (Eu 3+ -DTTA) in a vial with magnetic stirring at 28°C for 16 hours. The reaction solution was chromatographed on a Sephadex-G50 column (1×40 cm) equilibrated with 80 mmol / LTris-HCl pH 7.8 buffer solution to collect protein peaks, and diluted for use. ...
Embodiment 3
[0080] Embodiment 3 prepares kit
[0081] Preparation of OTA-KLH antigen
[0082] 1. Dissolve 2mg OTA with dimethylformamide (DMF);
[0083] 2. Use 0.13mol / L NaHCO 3 Dissolve 2mg of KLH carrier protein in coupling buffer;
[0084] 3. Add 0.8mg OTA solution to the KLH solution;
[0085] 4. Dissolve 4 mg of carbodiimide (EDC) in double distilled water, and add 100 μL to the above mixture, and let it act for 2 hours at room temperature (protect from light);
[0086] 5. After centrifugation, take the supernatant and separate it on a column (Sephadex G-25) for UV scanning detection.
[0087] The preparation of the polyclonal ochratoxin A antibody was the same as that in Example 1, with a few omissions.
[0088] Eu 3+ - Preparation of goat anti-rabbit antibody:
[0089] Take 2ml of 5g / L goat anti-rabbit antibody dissolved in 50mmol / L PBS pH7.0, change the buffer condition through PD-10 column, and the eluent is 50mmol / L NaCl containing 0.155mol / L NaCl 2 CO 3 -NaHCO 3 pH 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com