Time-resolved fluorescence (TRF) immunized detection kit of ovarian cancer tumor marker HE4
A time-resolved fluorescence, HE4 technology, applied in biological testing, anti-animal/human immunoglobulin, analytical materials, etc., can solve problems such as rising
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
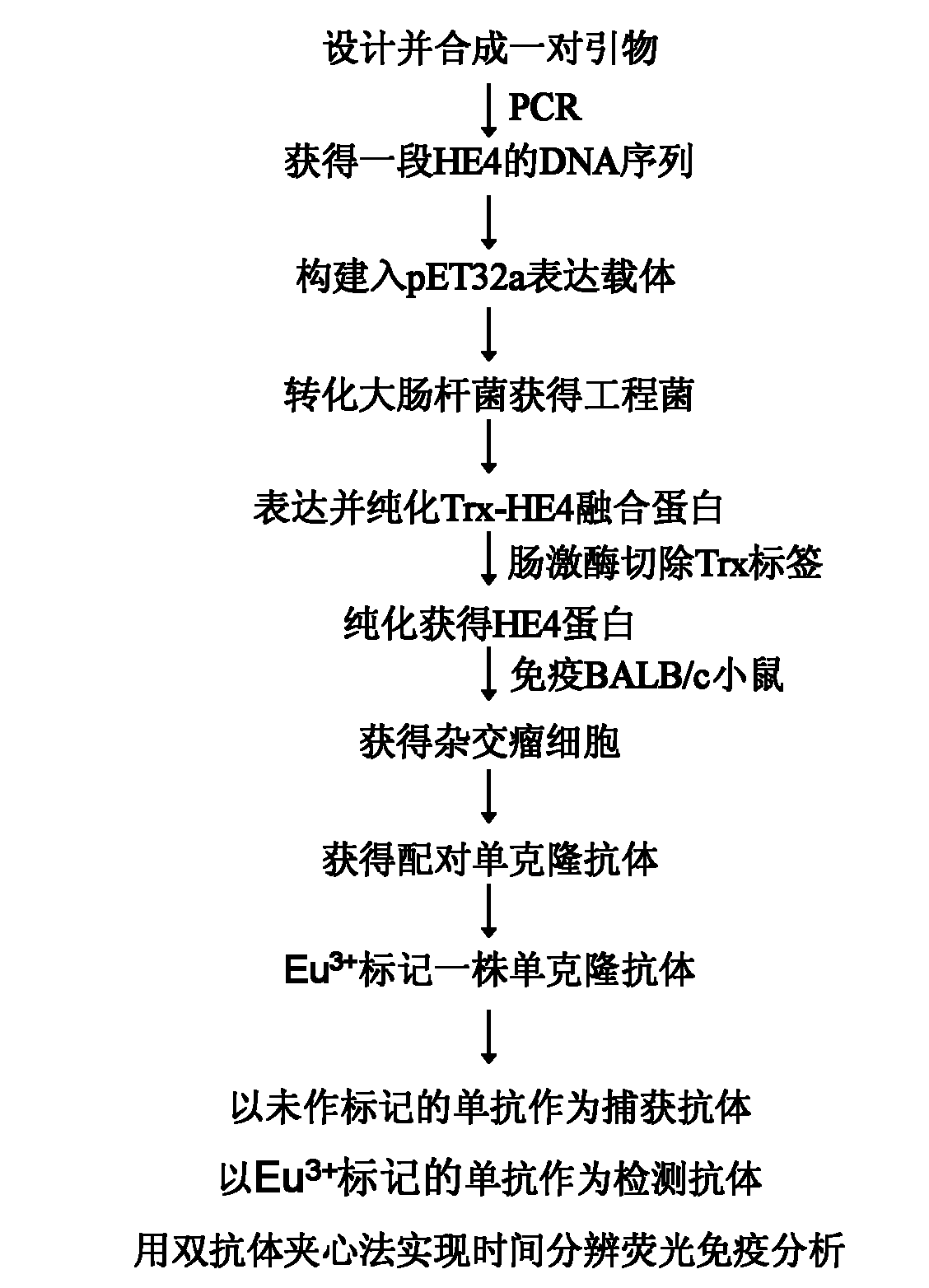
[0013] Example 1: Preparation of HE4 antigen
[0014] 1. Construction of pET-32a-HE4 recombinant vector
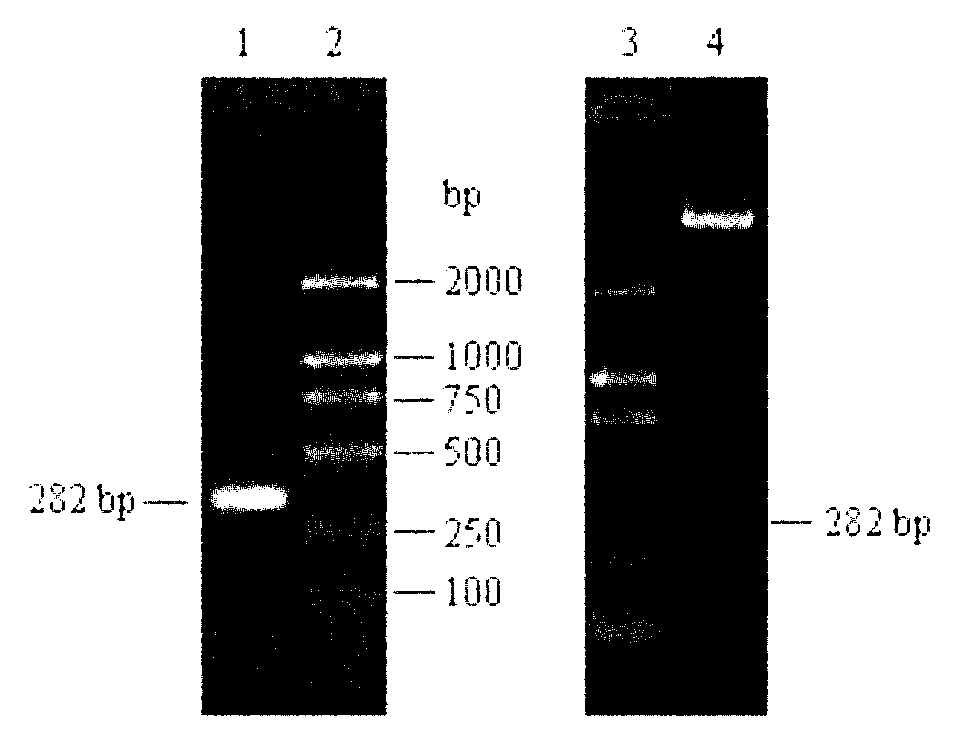
[0015] According to the human HE4 mRNA sequence (NM_006103) provided in GenBank, the N-terminal signal peptide was removed, a pair of primers were designed, and BamH I and Xho I restriction sites were introduced at the 5' ends of the two primers, respectively. The HE4 gene was extracted from the cancer cell line SKOV3, and agarose gel electrophoresis showed that the HE4 gene was successfully obtained, and the fragment length was consistent with the expectation ( figure 2 ).
[0016] The vector pET-32a(+) and the HE4 gene fragment purified by agarose gel were digested with BamH I and Xho I, and the digested product was purified and connected to obtain the recombinant plasmid pET-32a-HE4, the recombinant plasmid It was identified by double enzyme digestion ( figure 2 ), and the recombinant plasmid was sent to the company for sequencing. The sequencing results showed tha...
Embodiment 2
[0024] Example 2: Preparation of mouse anti-human HE4 monoclonal antibody
[0025] 1. Establishment of Hybridoma Cell Lines
[0026] Take 100μl (about 100μg) of purified HE4 protein and fully emulsify with the same amount of Freund's complete adjuvant, and inject BALB / c mice subcutaneously at multiple points on the back. The mice were injected subcutaneously at multiple points in the back, and then immunized once every two weeks. The immunization dose of each mouse was 50 μg without adjuvant. The immunization route was intraperitoneal injection. Starting from the second immunization, the tail vein was administered on the seventh day after each immunization The blood of mice was collected, and the serum titer reached 1:50,000 or more by indirect ELISA, and the mice were ready for fusion. Three days before fusion, a booster immunization was given by tail vein injection, and the antigen dose was 50 μg.
[0027] The mouse splenocytes were aseptically isolated during cell fusion, ...
Embodiment 3
[0034] Example 3: Establishment of Human Ovarian Cancer Tumor Marker HE4 Double Antibody Sandwich Time Resolved Fluorescence Immunoassay Method
[0035] 1. Detection method
[0036] In this example, the 6C2 monoclonal antibody obtained in Example 2 was used as the capture antibody, and Eu 3+ The labeled 5A3 monoclonal antibody was used as the detection antibody to establish a double-antibody sandwich time-resolved fluorescence immunoassay method for the detection of ovarian cancer tumor marker HE4.
[0037] 2. ELISA plate coating
[0038] Dilute the 6C2 monoclonal antibody to 10 μg / ml with coating solution (0.05mol / L carbonate buffer, pH 9.6), mix well, add 100 μl of the diluted monoclonal antibody solution to each well of the 96-well microtiter plate. The microtiter plate was sealed at 4°C overnight.
[0039] 3. ELISA plate blocking
[0040] PBS containing 20% fetal bovine serum was used as blocking solution. The ELISA plate coated overnight was patted dry, 200 μl of b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com