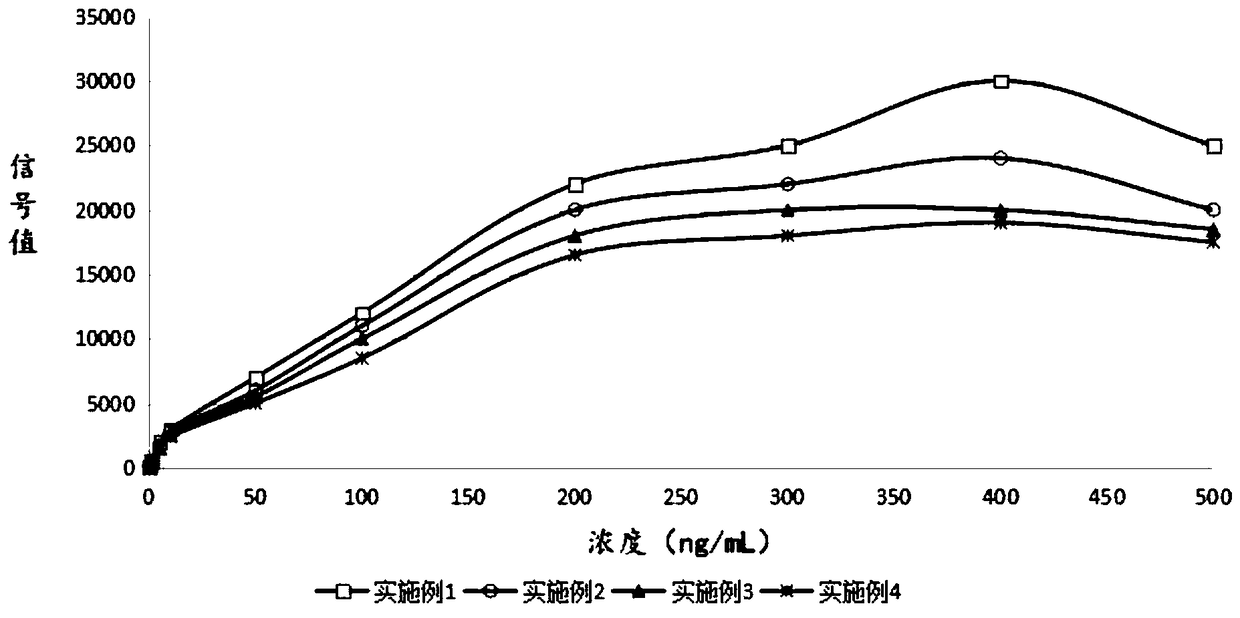
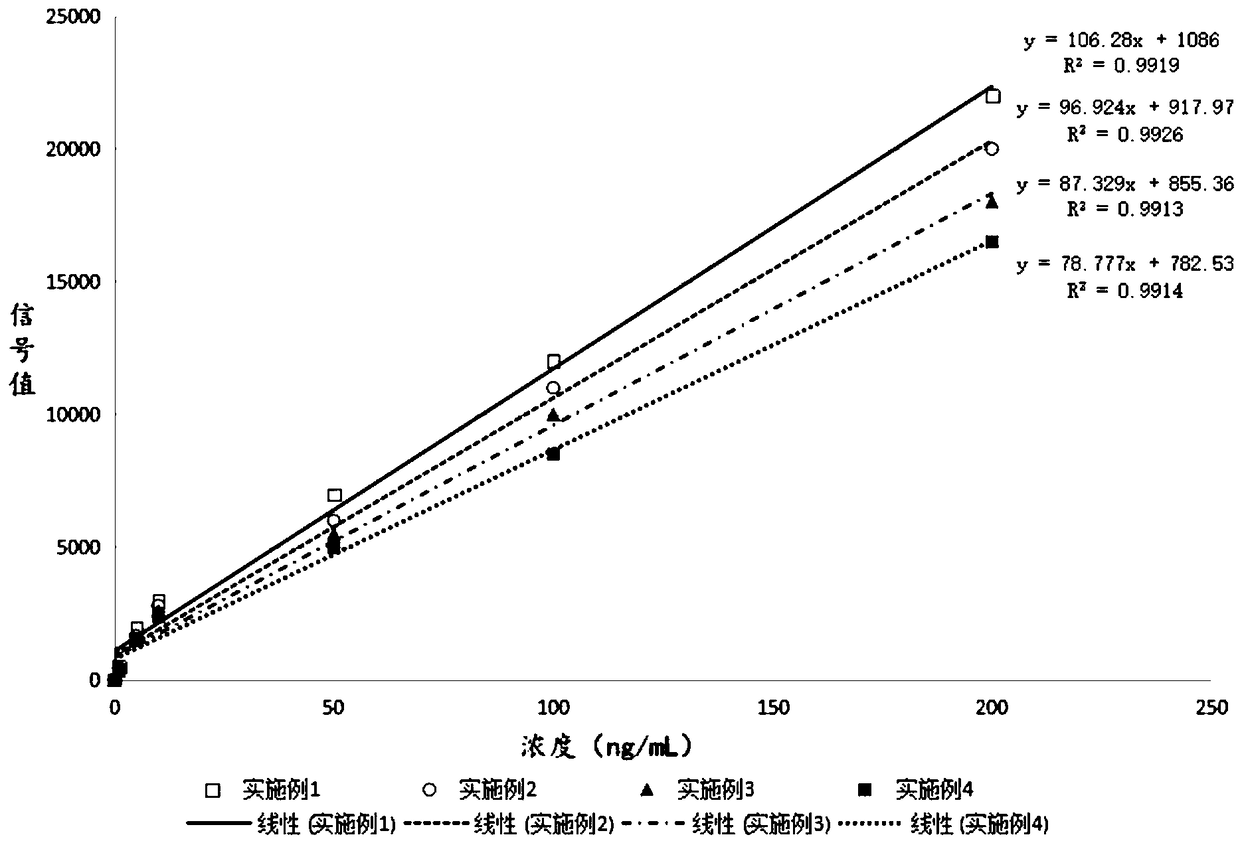
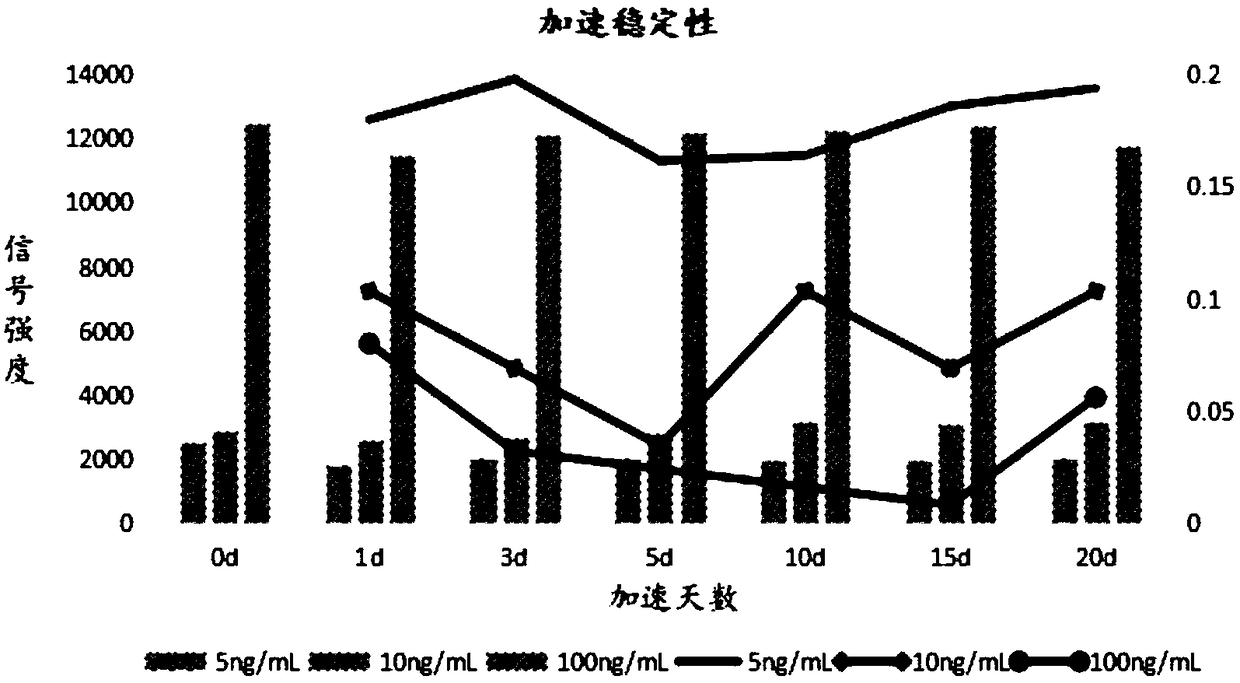
Time resolution fluorescence immunoassay method based on magnetic separation
A technology of time-resolved fluorescence and analysis methods, applied in the field of time-resolved fluorescence immunoassay analysis based on magnetic separation, can solve the problems of limited application development in the field of immunodiagnosis, easy to be interfered by environmental substances, short luminescence time, etc., to overcome the luminous intensity Weak, no radioactive contamination, wide standard curve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The specific operation steps of a time-resolved fluorescence immunoassay method based on magnetic separation in the present invention:
[0034] Step 1: After activation, the magnetic beads are coupled with monoclonal antibodies, blocked, and washed to obtain immunomagnetic beads, which are stored for later use; the specific steps are as follows:
[0035] Pretreatment: Take 25 μL of magnetic beads with 1% solid content and 1 μm surface carboxyl group modification in a 2 mL imported centrifuge tube, add 500 μL of 50 mmol / L MES solution with pH 6.0, vortex mix, centrifuge at 15000 rpm, 10 min, 4 °C, remove Add 500μL of 50mmol / L pH6.0 MES solution to the supernatant, and ultrasonically reconstitute;
[0036] Activation: Add 2 μL of 25mg / mL NHS (50mmol / L pH6.0 MES configuration), vortex mixing, then add 10mg / mL EDC (50mmol / L pH6.0 MES configuration) 2μL, vortex mixing, shaker at 250r / min, 15min at 37°C;
[0037] Coupling: 15000rpm, 10min, centrifuge at 4°C, remove superna...
Embodiment 2
[0059] The specific operation steps of a time-resolved fluorescence immunoassay analysis method based on magnetic separation of the present invention:
[0060] Step 1: After activation, the magnetic beads are coupled with monoclonal antibodies, blocked, and washed to obtain immunomagnetic beads, which are stored for later use; the specific steps are as follows:
[0061] Pretreatment: Take 25 μL of magnetic beads with 1% solid content and 2 μm surface carboxyl modification in a 2 mL imported centrifuge tube, add 500 μL of 20 mmol / L MES solution with pH6.0, vortex mix, centrifuge at 15000 rpm, 10 min, 4 °C, remove Add 500μL of 20mmol / L pH6.0 MES solution to the supernatant, and reconstitute by ultrasonication;
[0062] Activation: Add 2μL of 15mg / mL NHS (20mmol / L pH6.0 MES configuration), vortex to mix, then add 10mg / mL EDC (20mmol / L pH6.0 MES configuration) 2μL, vortex to mix, shake at 250r / min, 15min at 37°C;
[0063] Coupling: centrifuge at 15000rpm, 10min, 4°C, remove the...
Embodiment 3
[0085] The specific operation steps of a time-resolved fluorescence immunoassay analysis method based on magnetic separation of the present invention:
[0086] Step 1: After activation, the magnetic beads are coupled with monoclonal antibodies, blocked, and washed to obtain immunomagnetic beads, which are stored for later use; the specific steps are as follows:
[0087] Pretreatment: Take 25 μL of magnetic beads with 1% solid content and particle size of 500 nm surface hydroxyl modification in a 2 mL imported centrifuge tube, add 500 μL of 50 mmol / L MES solution with pH6.0, vortex mix, centrifuge at 15000 rpm, 10 min, 4 °C, remove Add 500μL of 50mmol / L pH6.0 MES solution to the supernatant, and ultrasonically reconstitute;
[0088] Activation: Add 2μL of 25mg / mL NHS (50mmol / L pH6.0 MES configuration), vortex mixing, then add 10mg / mL EDC (50mmol / L pH6.0 MES configuration) 2μL, vortex mixing, shaker 100r / min, 30°C for 30min;
[0089] Coupling: 15000rpm, 10min, centrifuge at 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com