Methods for measuring platelet reactivity of patients that have received drug eluting stents
a technology for elcosanoid stents and platelet aggregation, which is applied in the field of diagnostic assays, can solve the problems of inability to meet the needs of patients, inconvenient clinical environment, and inability to achieve optimal selection, so as to reduce the ability to form platelet thrombosis, enhance and enhance the effect of the signal transduction pathway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0058] The following examples are offered by way of illustration and without limitation. Parts and percentages are by weight unless otherwise indicated
[0059] The following examples and preparations are intended to illustrate the invention but are not intended to limit its scope.
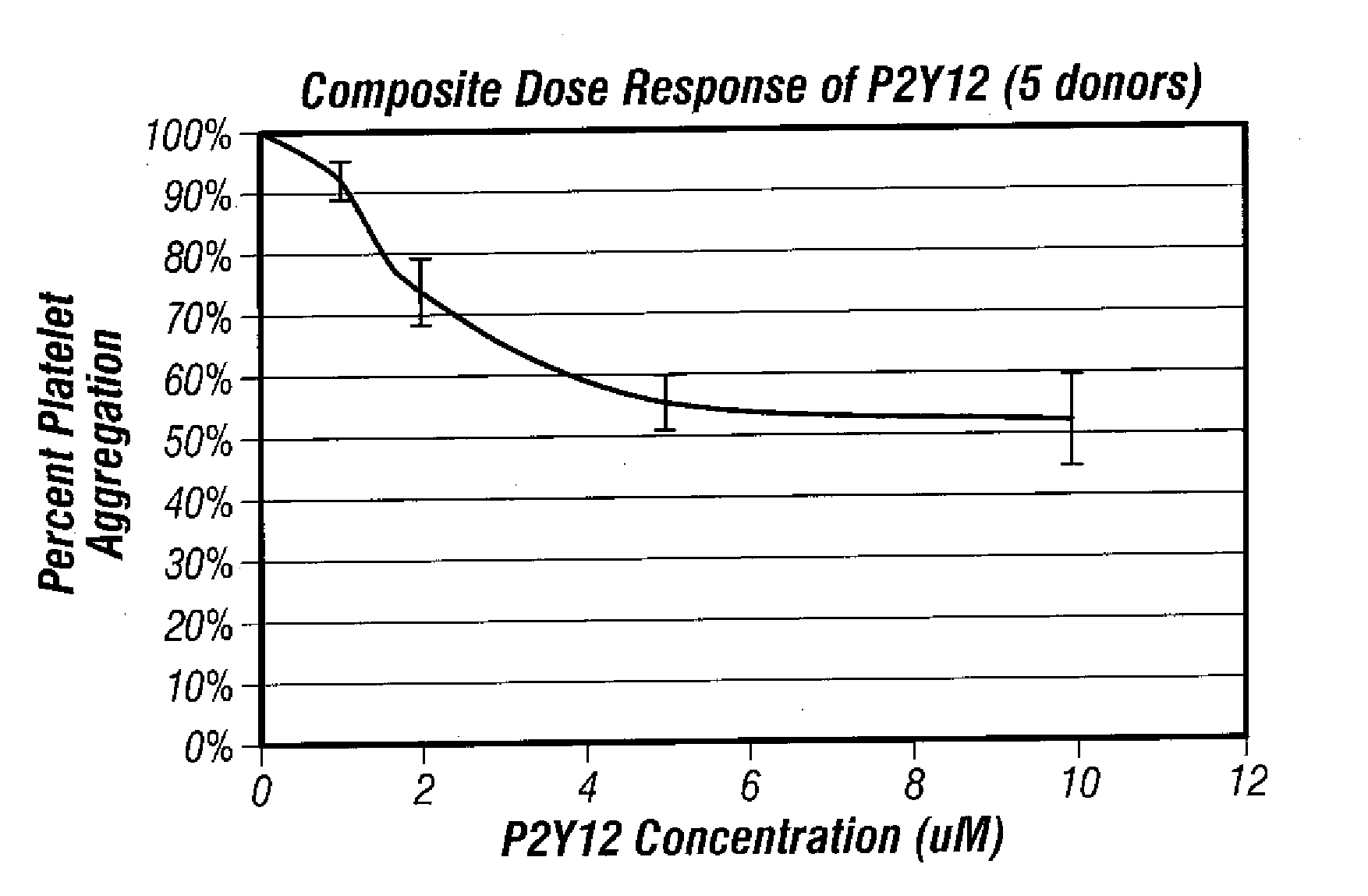
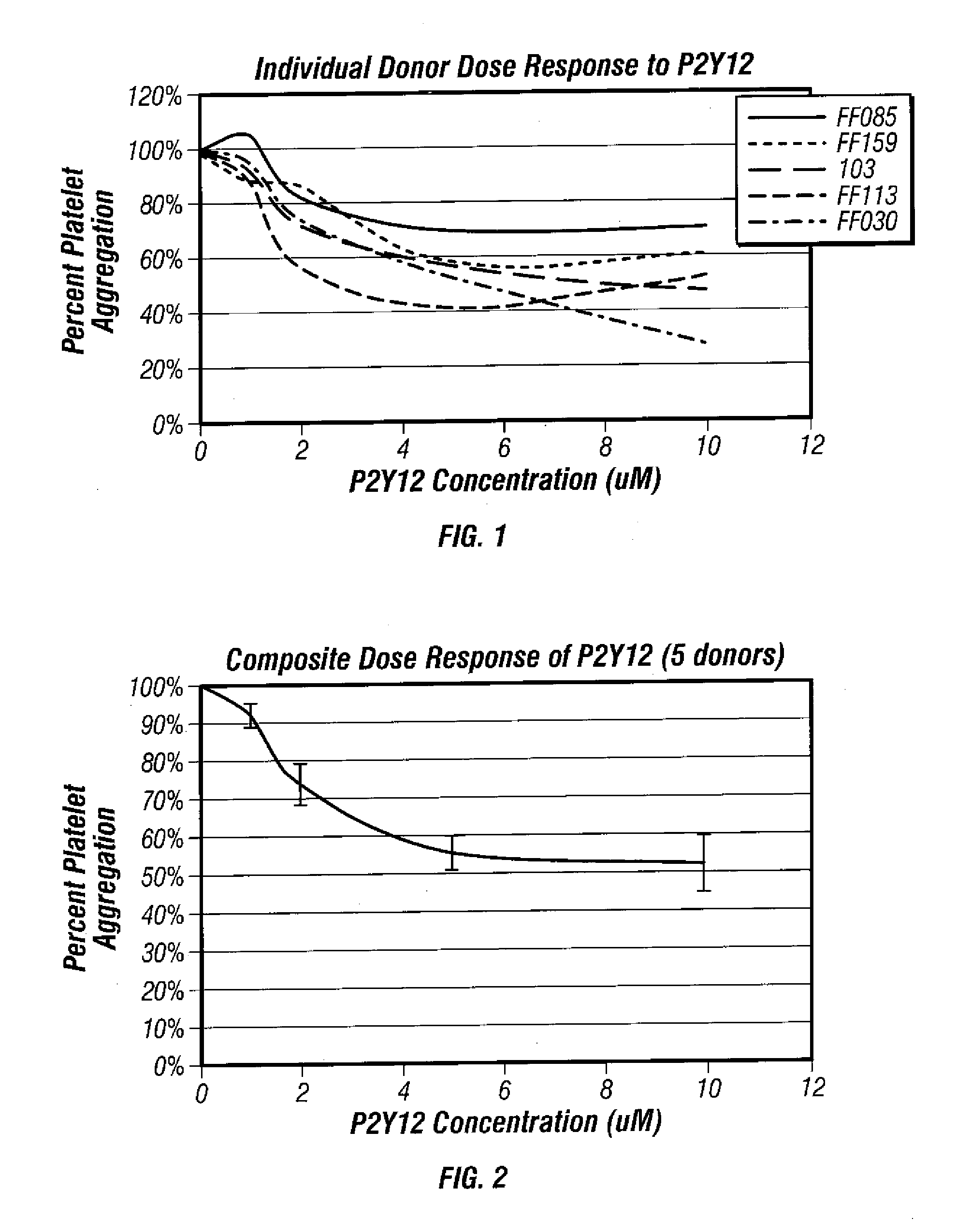
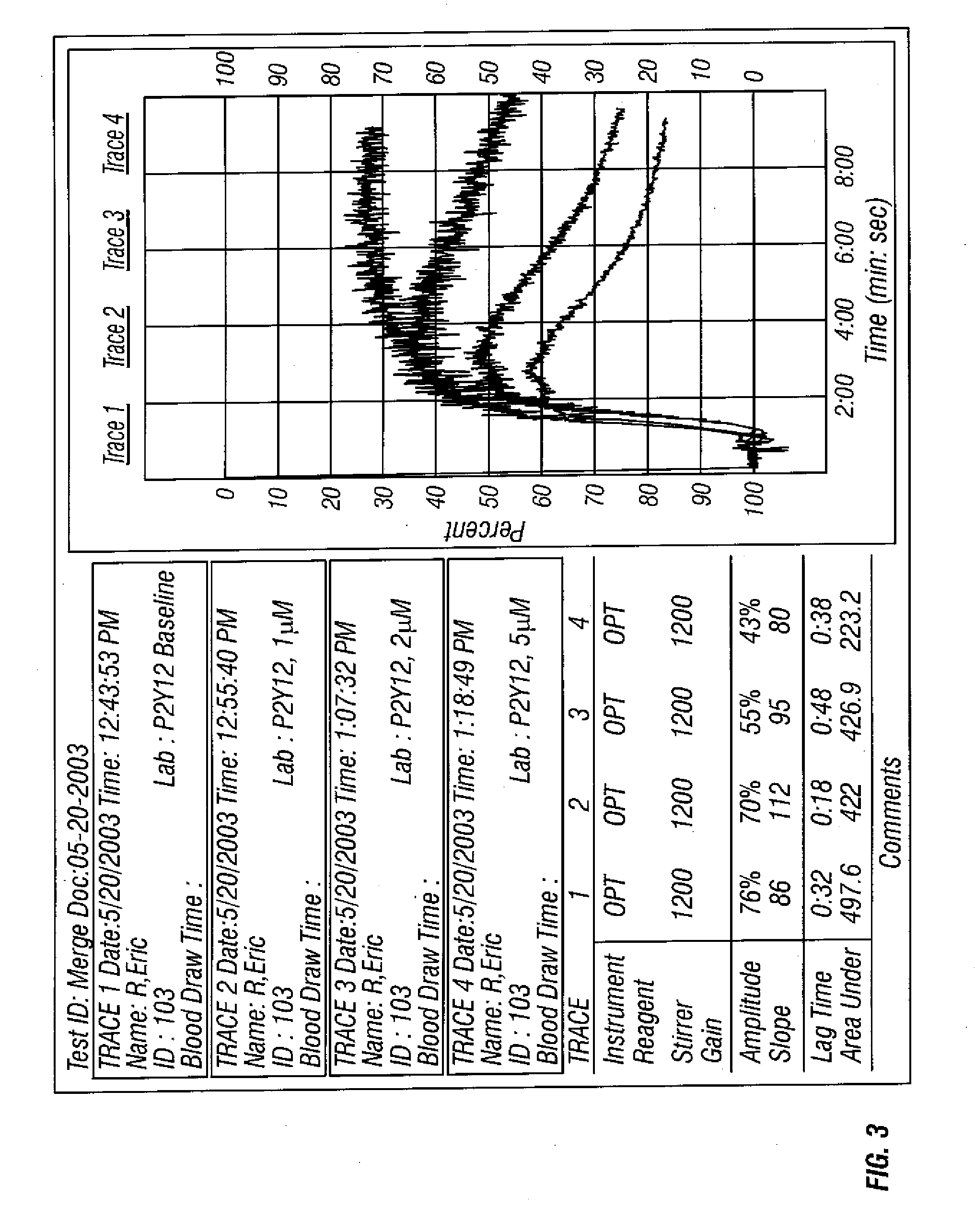
[0060] Dose response testing was performed withADP (Chronolog) and PGE1 (SIGMA) at 20 μM and 22 nM final concentrations respectively. ADP was diluted in Hepes / Saline, pH 7.4 buffer to a final concentration of 200 μM prior to use on the aggregometer. PGE1 was diluted in Hepes / Saline, pH 7.4 buffer to a final concentration of 220 nM prior to use on the aggregometer. A P2Y12 receptor blocker was diluted in DMF to final concentrations of 1 mM, 2 mM and 5 mM.
[0061] Five microliters of the diluted P2Y12 compound were spiked into 5 mL whole blood. Samples were inverted and incubated for one hour at room temperature. The whole blood baseline sample did not receive any additive. Once incubation was complete, whole ...
example 2
[0068] A study of PCI patients was conducted. The patients selected had at least one of the following, at least one lesion ≧50% diameter stenosis requiring PCI, a reference vessel diameter between 2.25 and 4.0 mm, target lesion located within a native coronary artery or bypass graft that was either de novo or restenotic and no known allergy to aspirin, clopidogrel, or any component of an DES.
Measurement of Platelet Reactivity
[0069] Whole blood was obtained from the sideport of the arterial sheath prior to anticoagulant or anti-thrombin therapy and by phlebotomy 12 hours post-PCI. The inhibitory effect of clopidogrel was measured using the VerifyNow® P2Y12 assay (Accumetrics, San Diego, Calif.). The percentage inhibition induced by clopidogrel in patients with a baseline sample prior to clopidogrel exposure was calculated using the formula:
1−(PRU post-clopidogrel / PRU baseline)×100.
[0070] If the post-treatment ADP-induced aggregation was greater after clopidogrel than at baseline,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com