Patents
Literature
1712results about How to "Reduce data" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
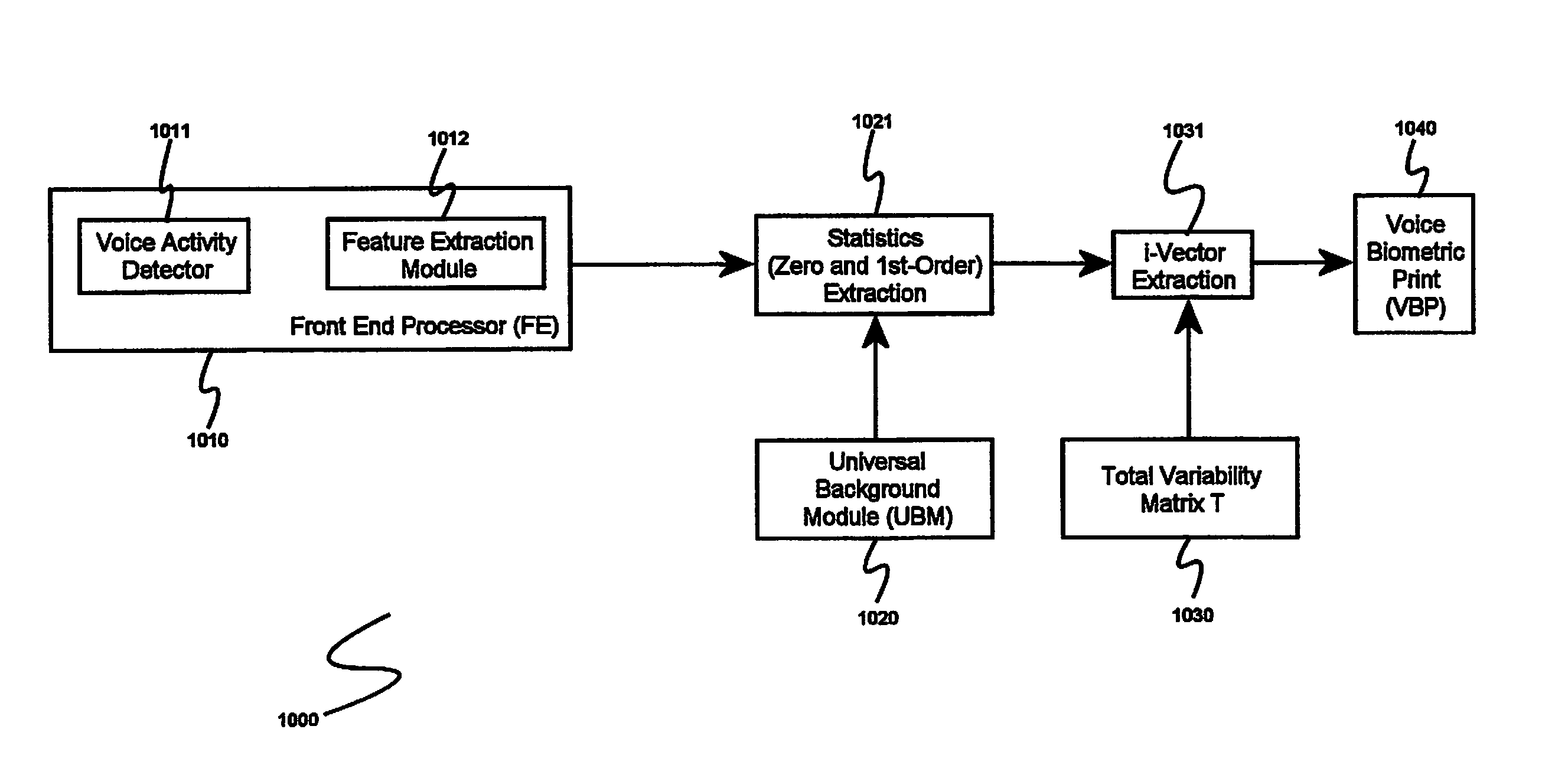
System and method for speaker recognition on mobile devices
InactiveUS20130225128A1Reduce storageReduce processUnauthorised/fraudulent call preventionEavesdropping prevention circuitsSpeaker recognition systemMobile device
A speaker recognition system for authenticating a mobile device user includes an enrollment and learning software module, a voice biometric authentication software module, and a secure software application. Upon request by a user of the mobile device, the enrollment and learning software module displays text prompts to the user, receives speech utterances from the user, and produces a voice biometric print. The enrollment and training software module determines when a voice biometric print has met at least a quality threshold before storing it on the mobile device. The secure software application prompts a user requiring authentication to repeat an utterance based at least on an attribute of a selected voice biometric print, receives a corresponding utterance, requests the voice biometric authentication software module to verify the identity of the second user using the utterance, and, if the user is authenticated, imports the voice biometric print.
Owner:CIRRUS LOGIC INC
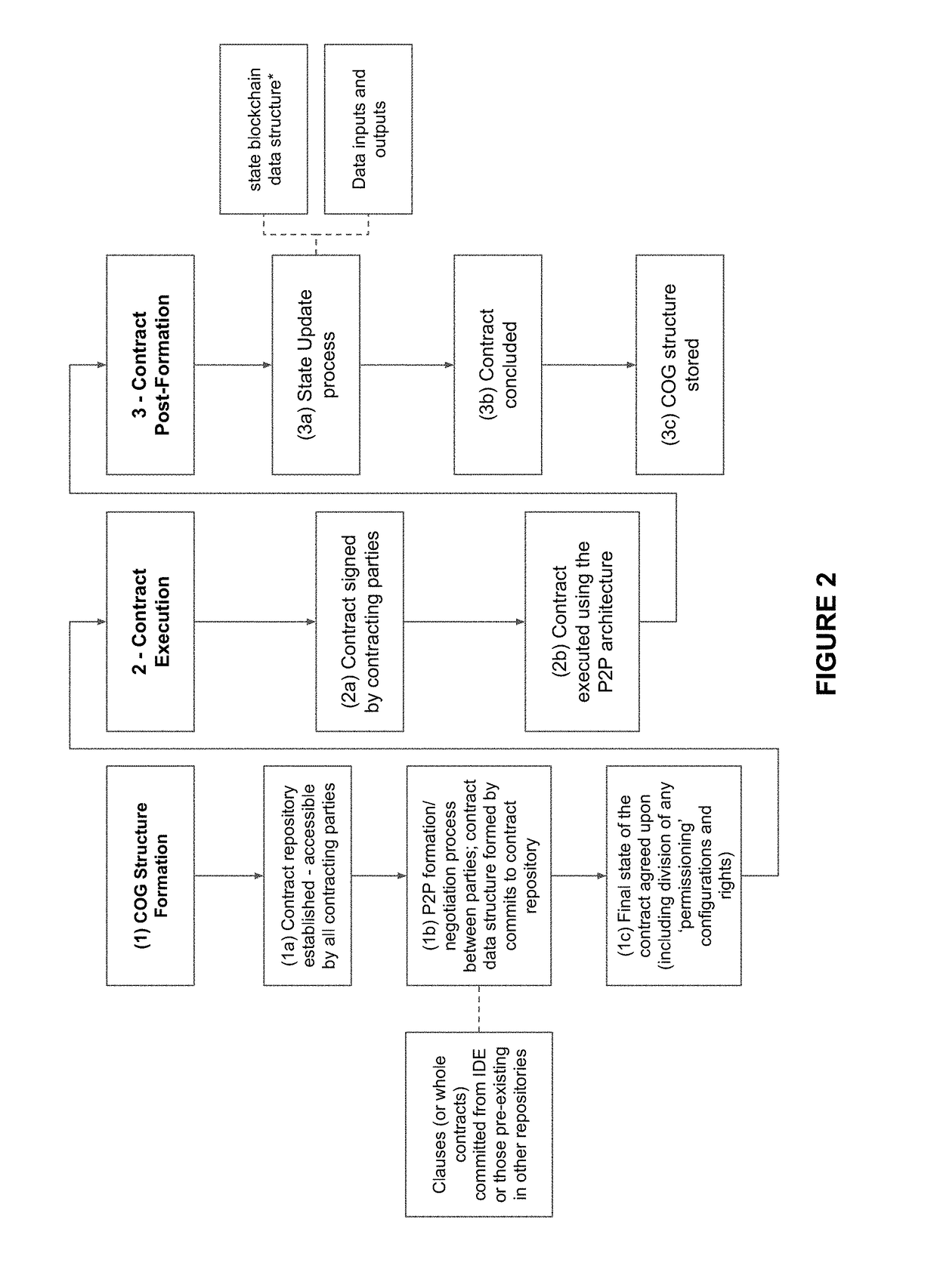
System and method for forming, storing, managing, and executing contracts
ActiveUS20180005186A1Efficiently and verifiably moveReduce dataDrawing from basic elementsCryptography processingDocumentationObject graph
A system and method for computable contracts that includes a contract management system accessible by involved parties, managing a formation stage of a contract document by obtaining object components, assembling a contract object graph from the object components, and committing the contract object graph to post formation execution; and in an execution environment during a post-formation stage, executing the contract object graph where instances of execution include receiving a contract state update, and appending at least one update object component to the contract object graph in accordance with the contract state update. Variations of the system and method may apply peer-to-peer negotiation and execution, use a cryptographic directed acyclic contract object graph, and / or interface with distributed ledgers.
Owner:DOCUSIGN
Method and system for extracting and classifying geolocation information utilizing electronic social media
InactiveUS20130086072A1DistanceReduce “ noisy ” dataDigital data information retrievalDigital data processing detailsData ingestionGeotargeting
Methods, systems and processor-readable media for extracting and classifying location information utilizing social media messages and / or data thereof. The social media messages can be sampled from a social media database and the messages filtered based on a heuristic rule. A geolocation entity from the unstructured social media messages can be extracted utilizing a geolocation entity extracting module. The messages with the geoentities can be uploaded onto a crowd sourcing platform to manually annotate the messages with a label. A text classification model can be built and learned from the label utilizing a machine learning algorithm and the messages can be classified by a location classifier in order to extract the user location. The user location can then be transformed into a geocode so that a spatial search can be enabled and the distance between the locations can be easily calculated.
Owner:XEROX CORP
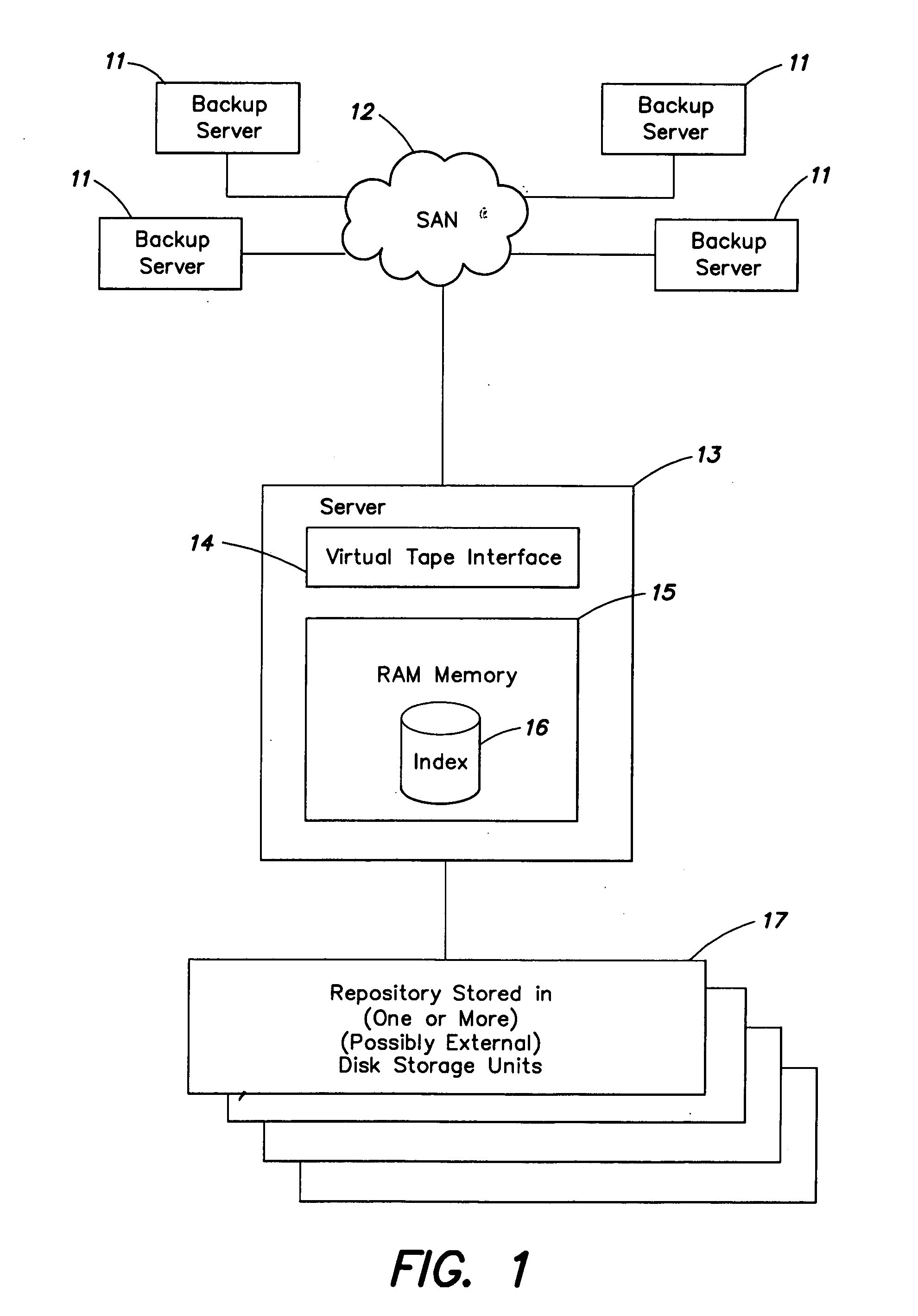
Systems and methods for efficient data searching, storage and reduction
InactiveUS20060059173A1Efficiently determinedReduce dataData processing applicationsDigital data information retrievalData segmentTheoretical computer science
Owner:IBM CORP
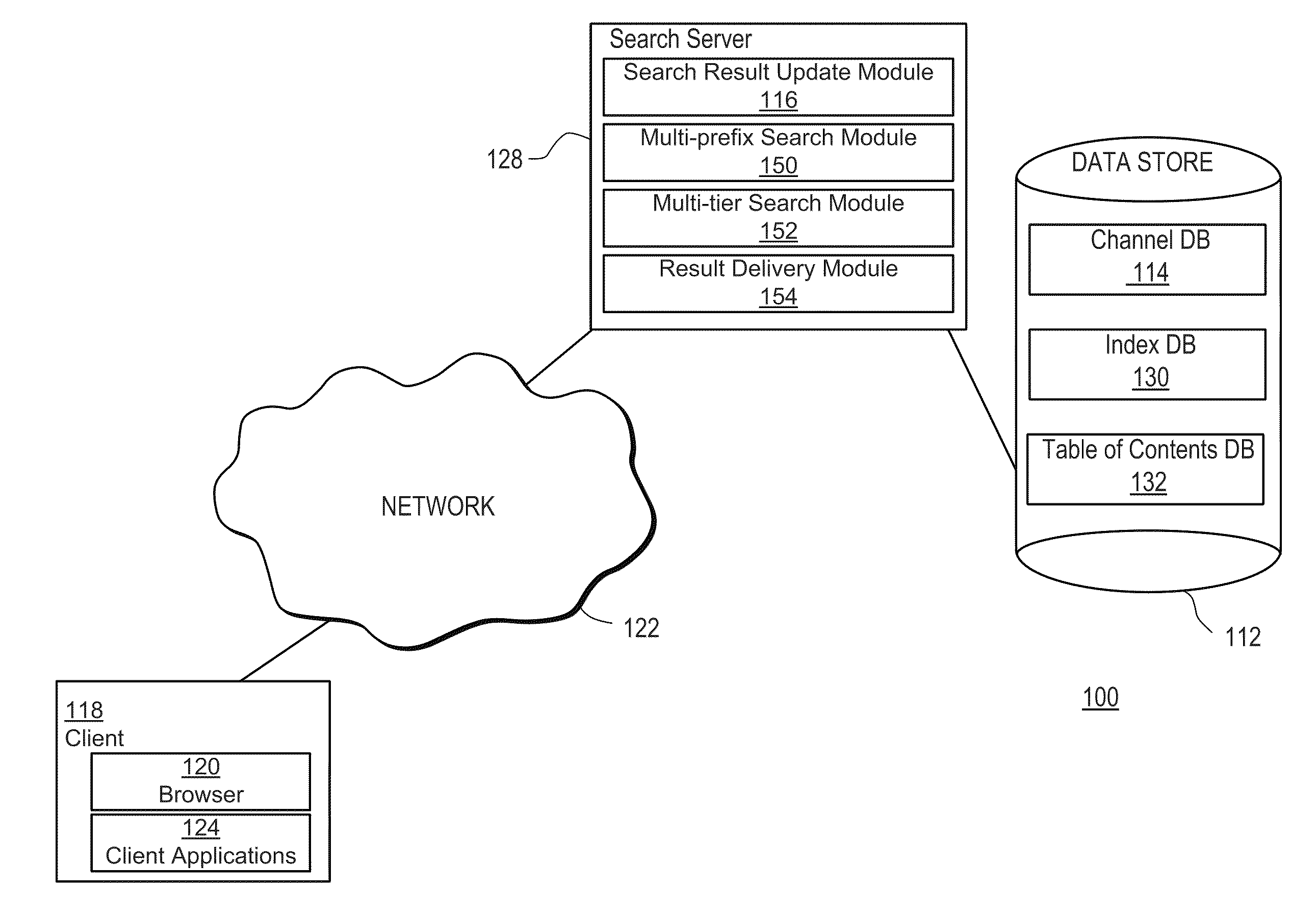
Leveraging Collaborative Cloud Services to Build and Share Apps
ActiveUS20110083167A1Facilitate targeted searchFacilitates targeted mobile searchesDigital data information retrievalDigital data processing detailsApplication softwareContext specific
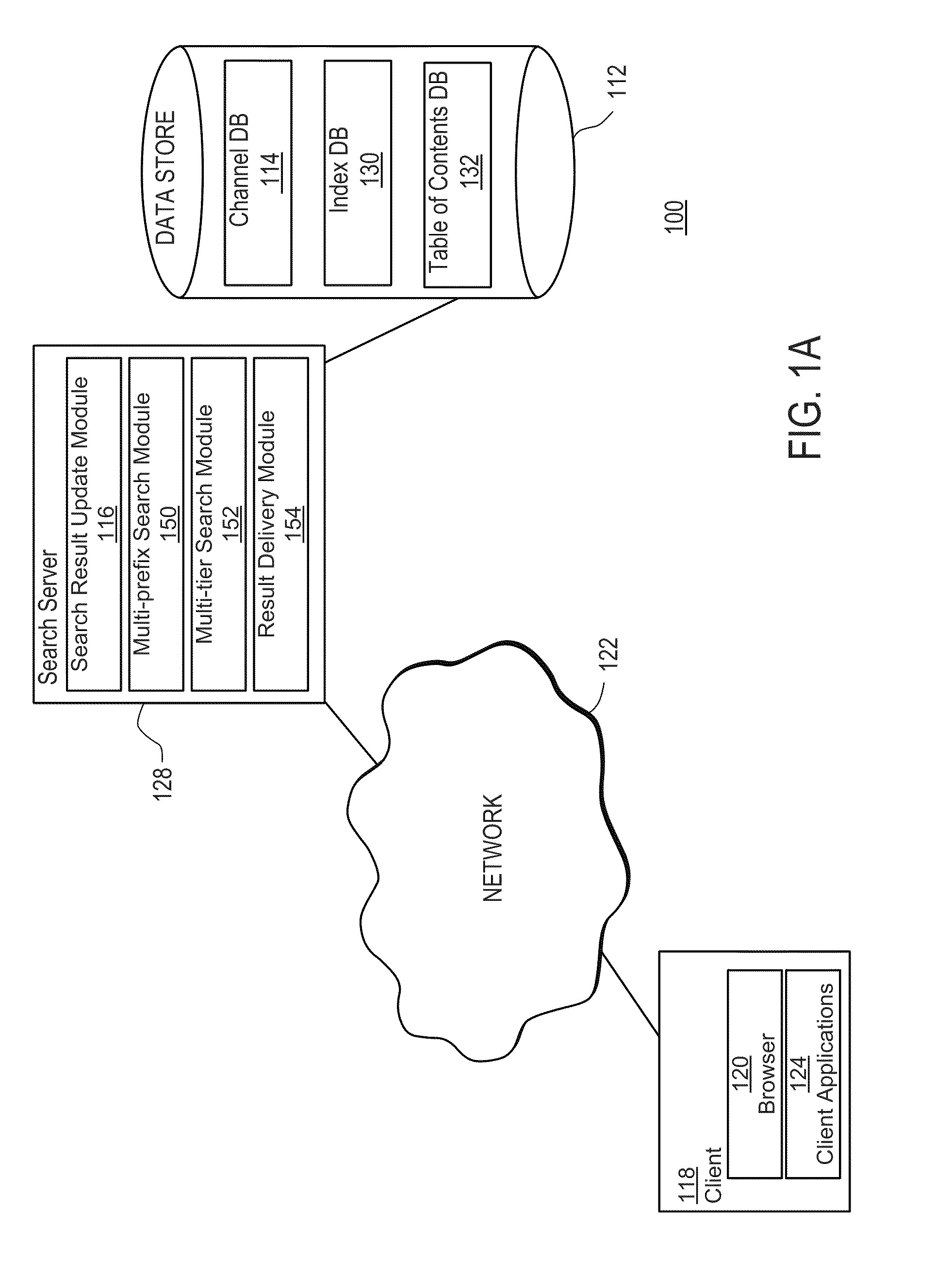
The present invention includes systems and methods for retrieving information via a flexible and consistent targeted search model that employs interactive multi-prefix, multi-tier and dynamic menu information retrieval techniques (including predictive text techniques to facilitate the generation of targeted ads) that provide context-specific functionality tailored to particular information channels, as well as to records within or across such channels, and other known state information. Users are presented with a consistent search interface among multiple tiers across and within a large domain of information sources, and need not learn different or special search syntax. A thin-client server-controlled architecture enables users of resource-constrained mobile communications devices to locate targeted information more quickly by entering fewer keystrokes and performing fewer query iterations and web page refreshes, which in turn reduces required network bandwidth. Applications are built by leveraging existing collaborative cloud services that enable the maintenance and sharing of user content.
Owner:BOOPSIE INC
Disk drive data caching using a multi-tiered memory
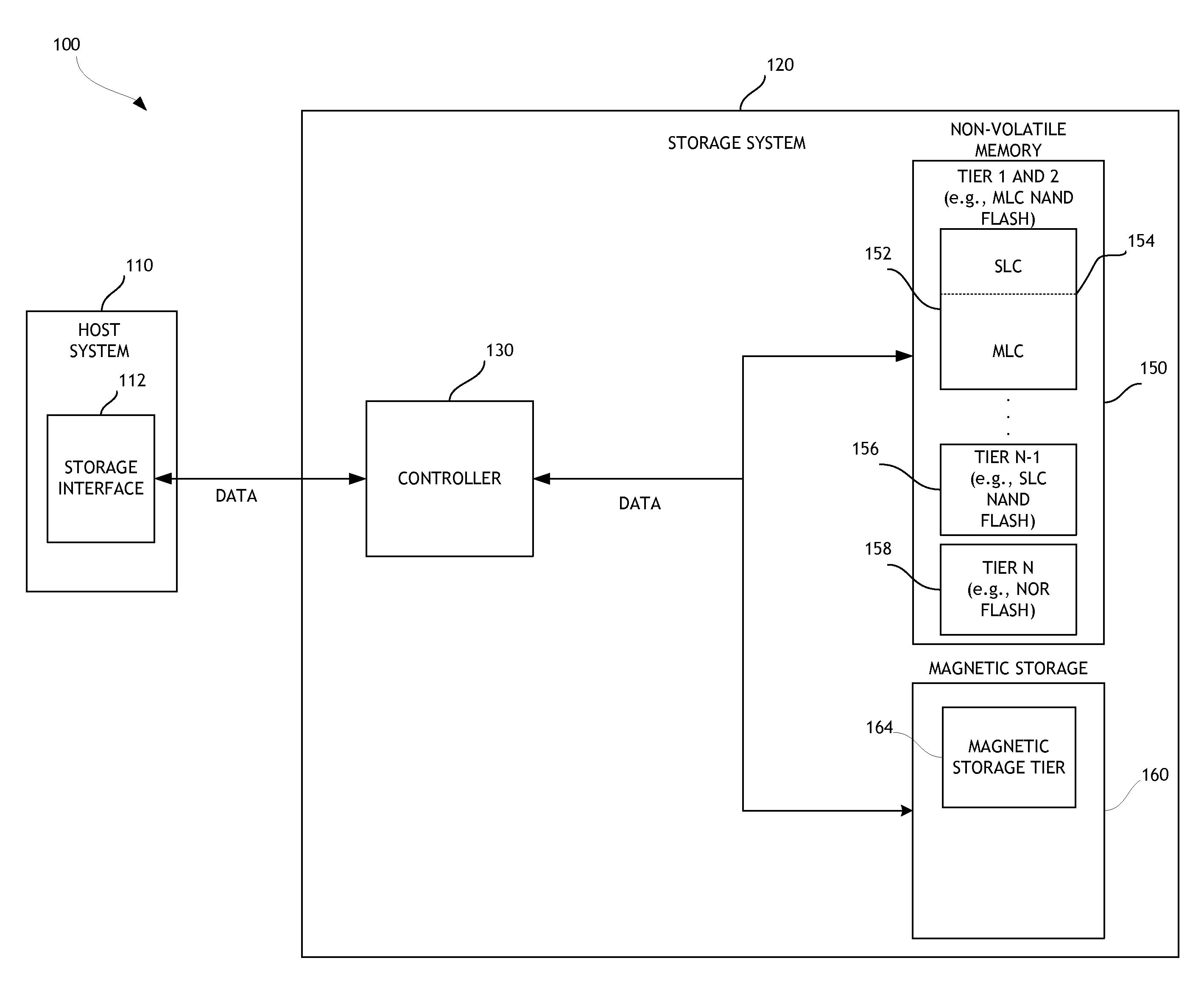
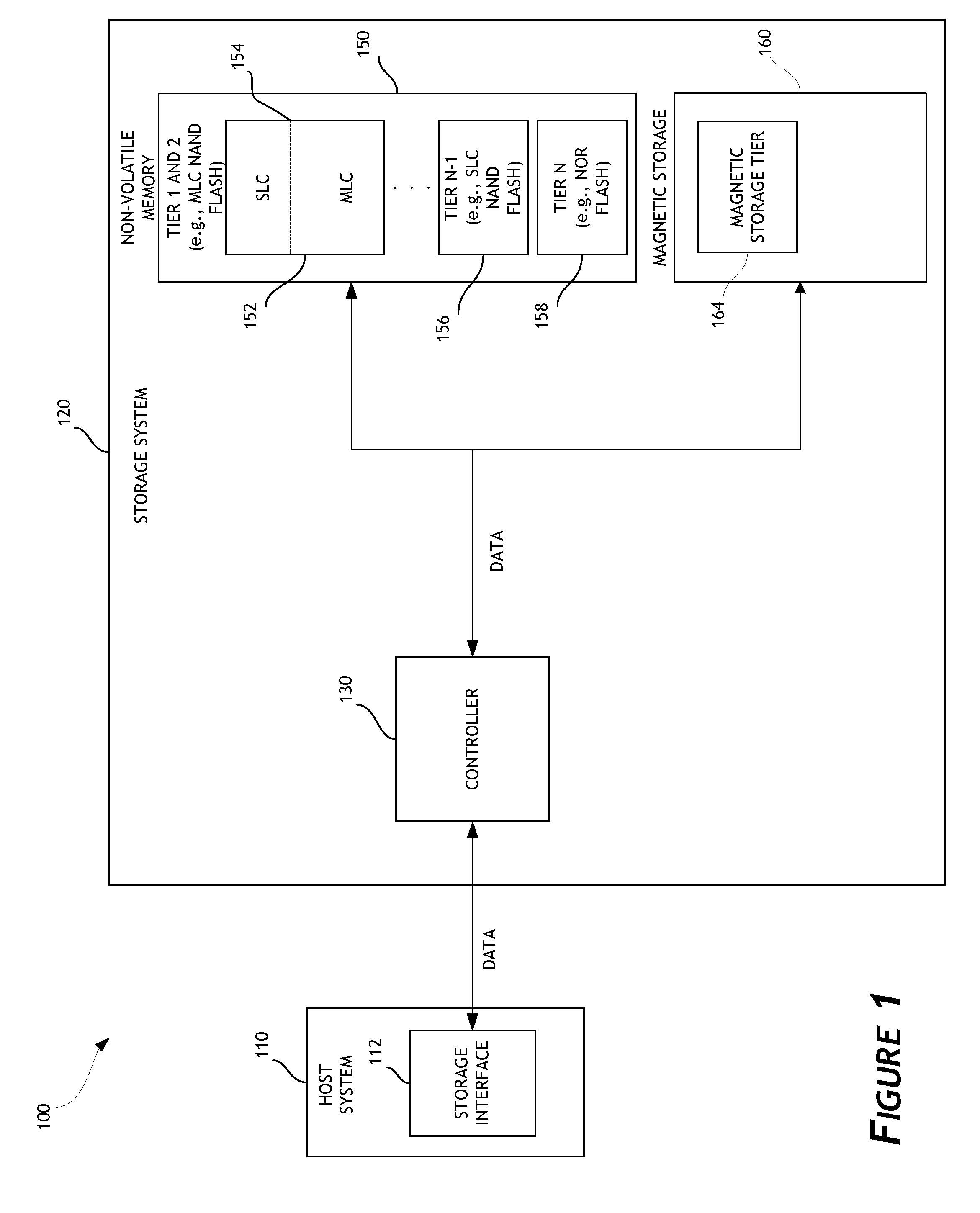
ActiveUS20130132638A1Limit usable lifeReduce dataMemory architecture accessing/allocationEnergy efficient ICTSingle levelComputer science
A disk drive is disclosed that utilizes multi-tiered solid state memory for caching data received from a host. Data can be stored in a memory tier that can provide the required performance at a low cost. For example, multi-level cell (MLC) memory can be used to store data that is frequently read but infrequently written. As another example, single-level cell (SLC) memory can be used to store data that is frequently written. Improved performance, reduced costs, and improved power consumption can thereby be attained.
Owner:WESTERN DIGITAL TECH INC
Manufacturing method of semiconductor integrated circuit device
InactiveUS20050090120A1Reduce dataSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusSemiconductorLight transmission
In a massed region of each of a plurality of transfer areas of a mask a plurality of light transmission patterns are formed by opening a half-tone film. A phase shifter is disposed in each of the light transmission patterns so that a 180° phase inversion occurs between the lights that transmit through adjacent light transmission patterns. In a sparse region of the plurality of transfer areas a solitary light transmission pattern is formed by opening the half-tone film. Both shape and size are the same among the light transmission patterns, which are disposed symmetrically in both the massed and sparse regions about the center between the transfer areas. The phase shifters in the massed regions are disposed so that the phase of each phase shifter in one of the transfer areas comes to be opposed to that of its counterpart in the other transfer area. In the exposure process, those transfer areas are overlaid one upon another in the same chip region.
Owner:HITACHI LTD
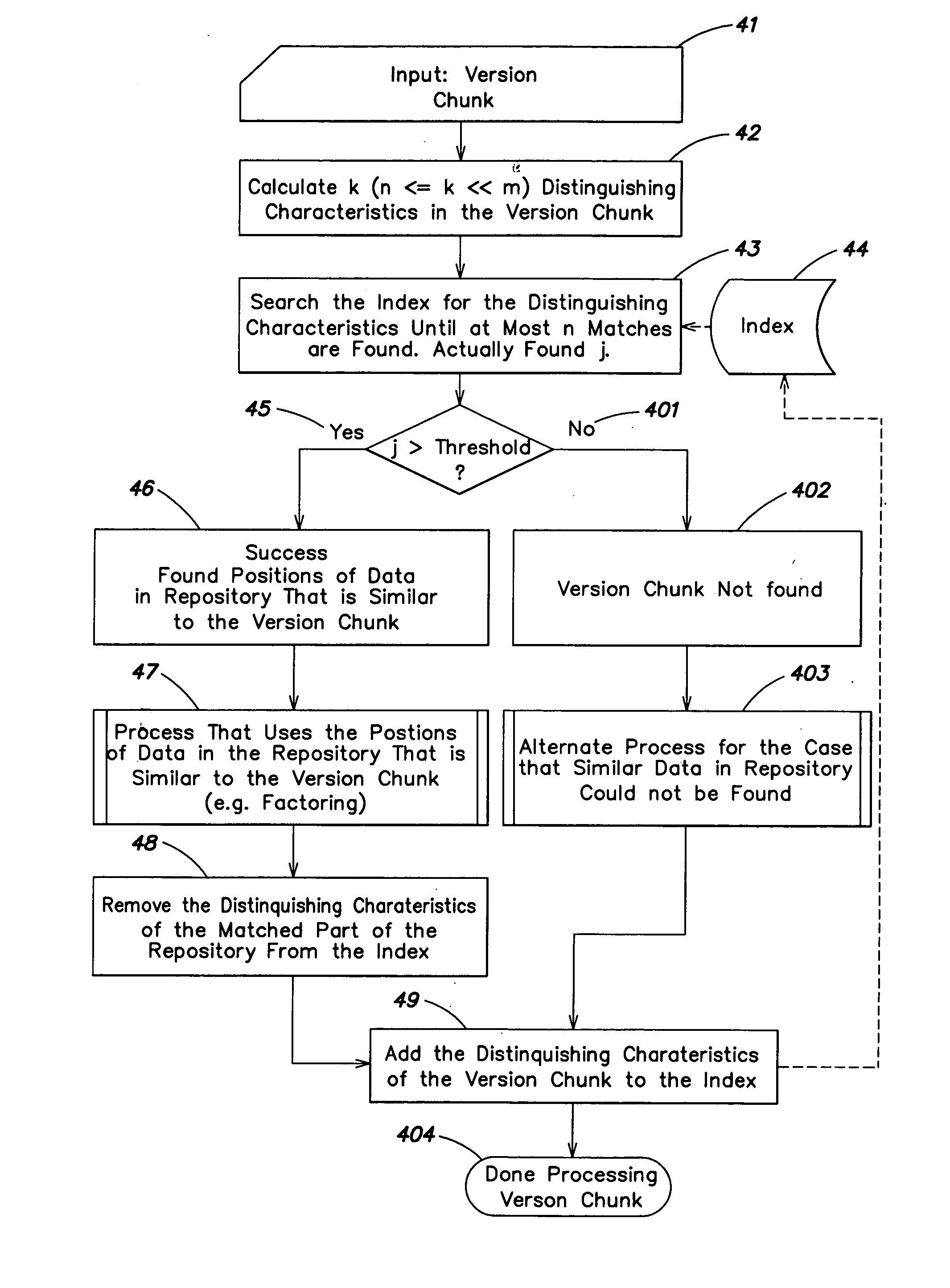
Multi-channel digital wireless audio system
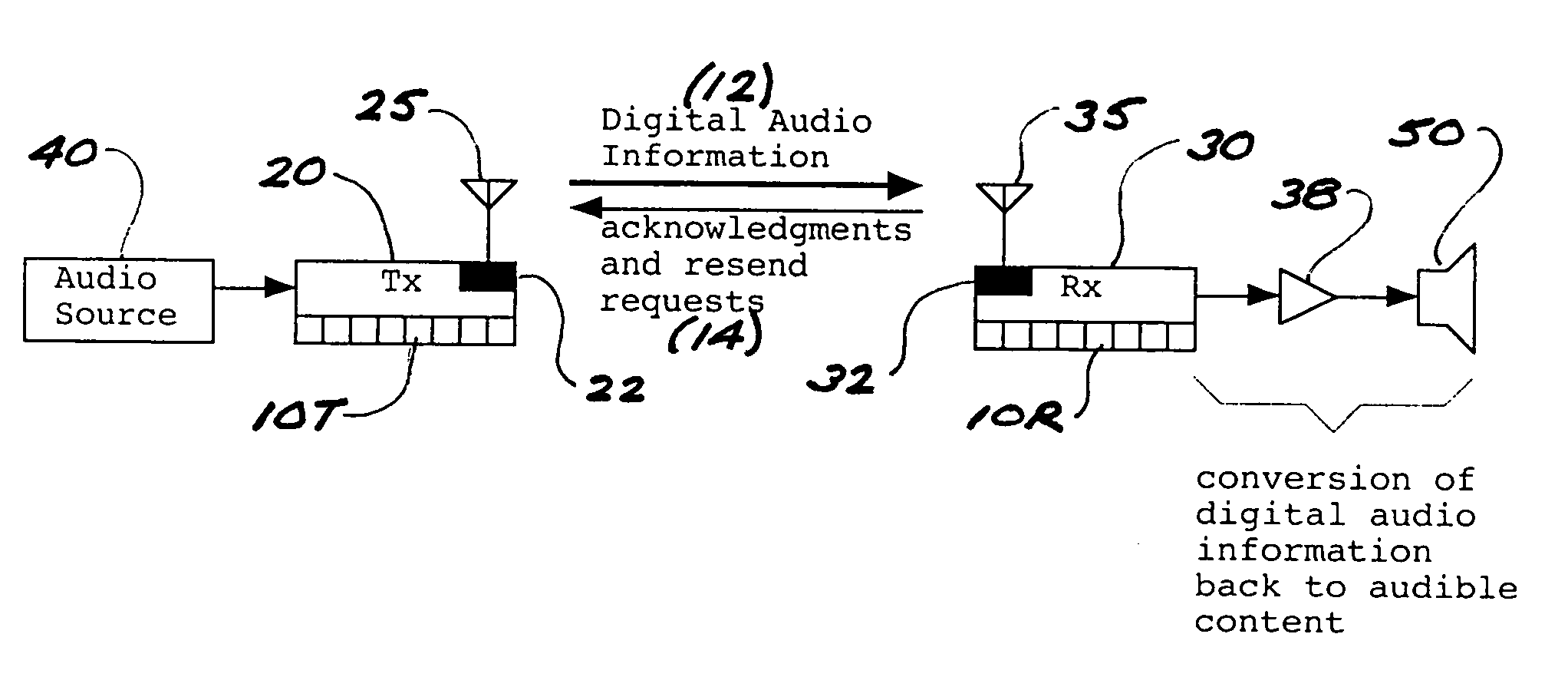
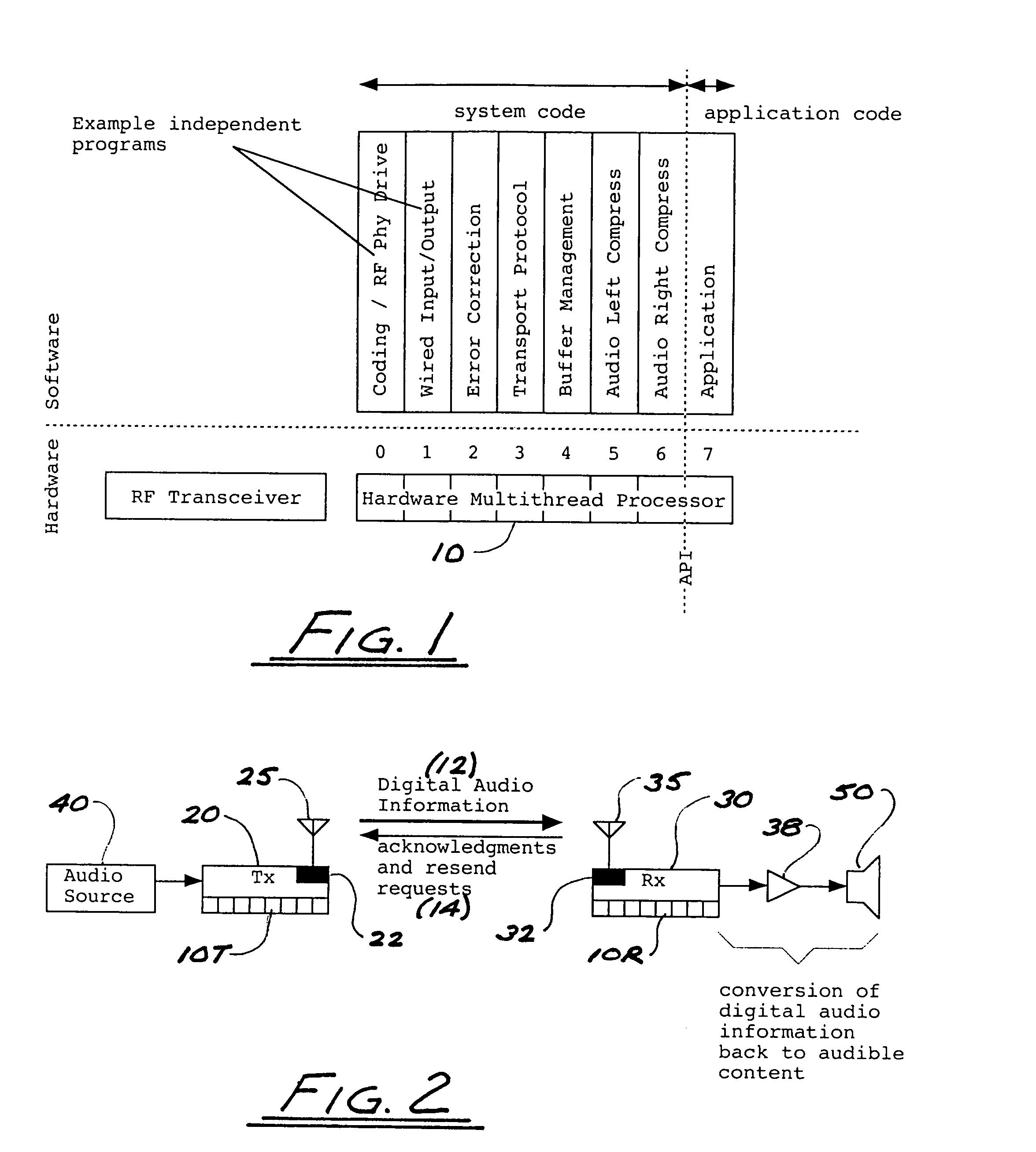
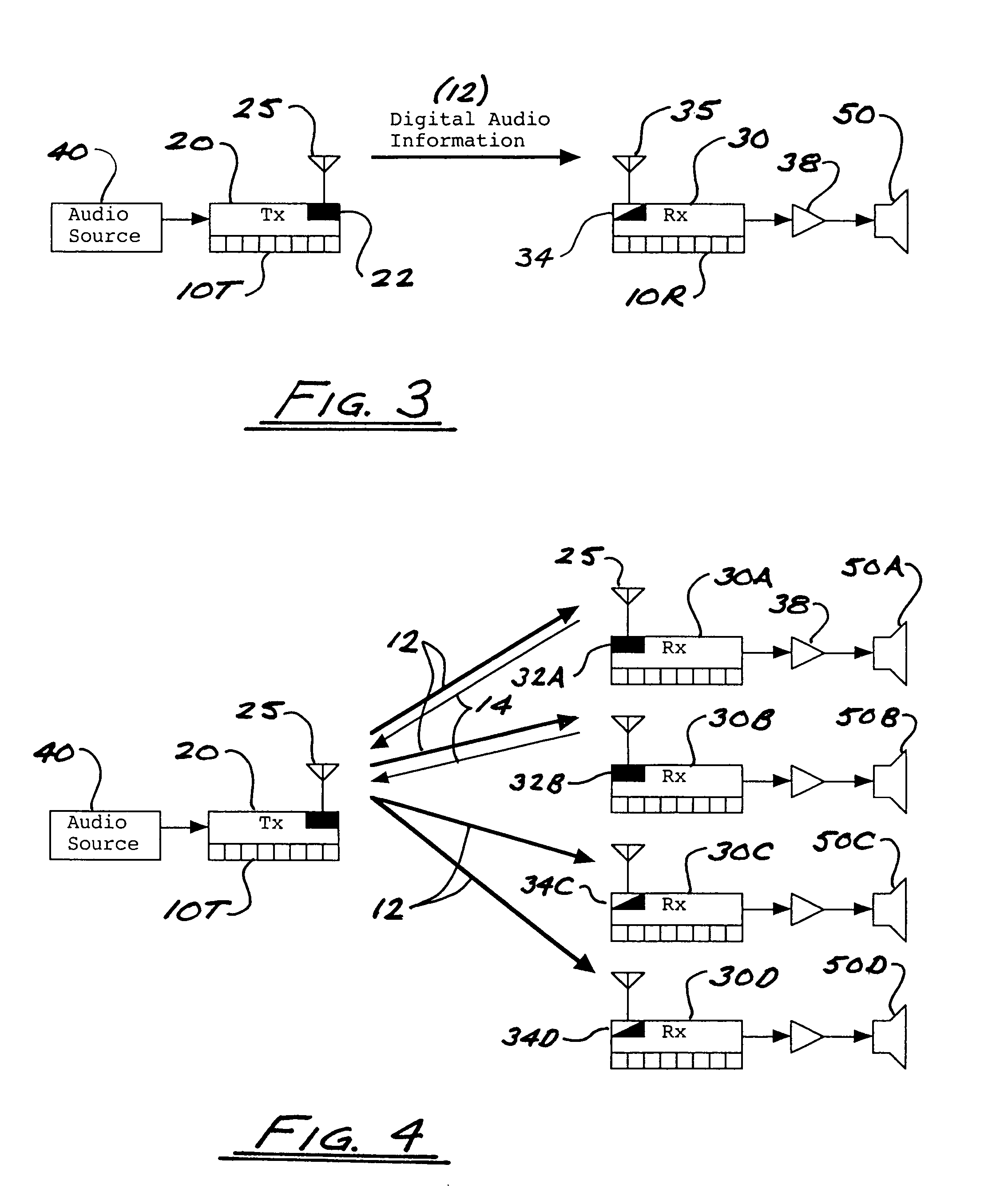
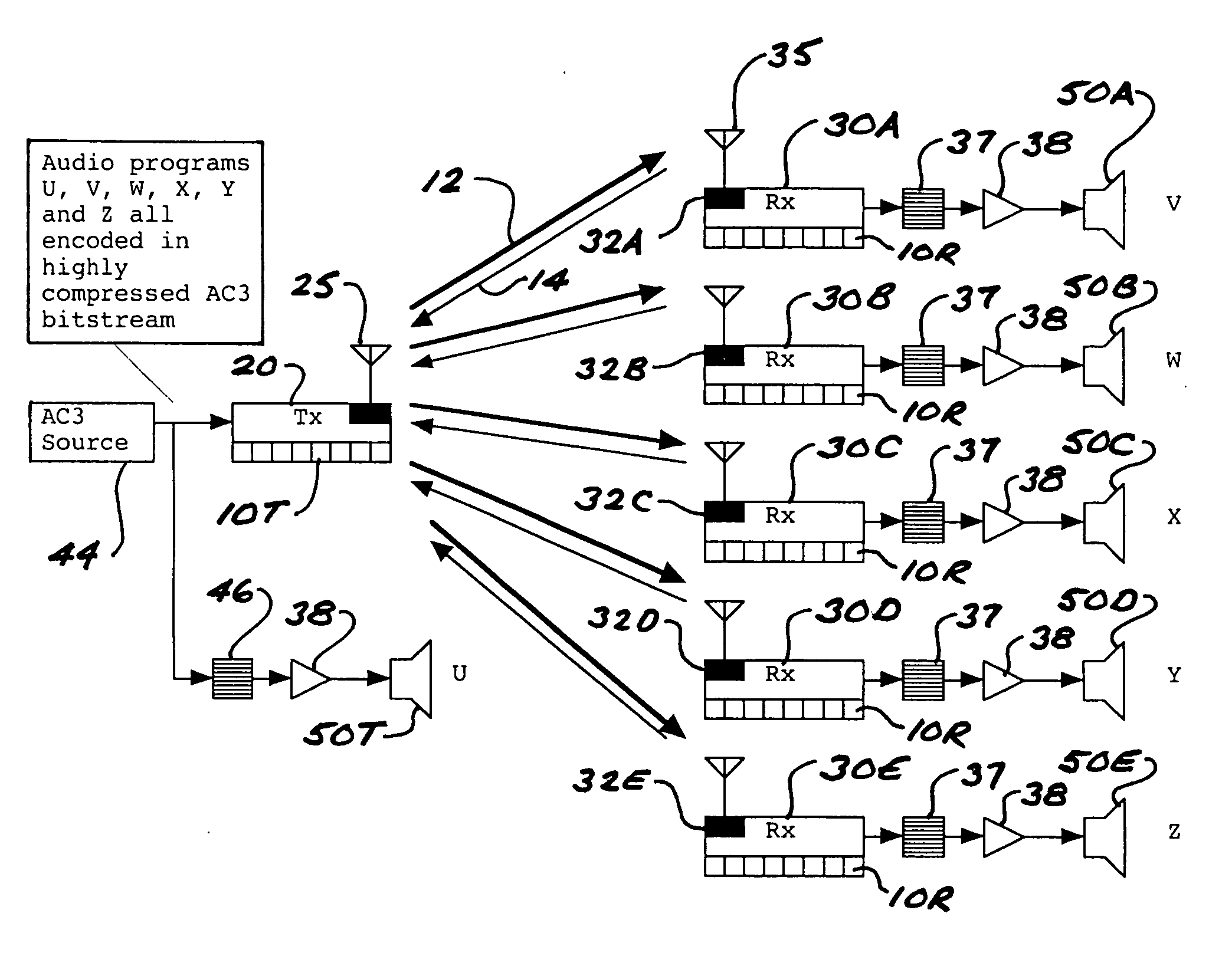
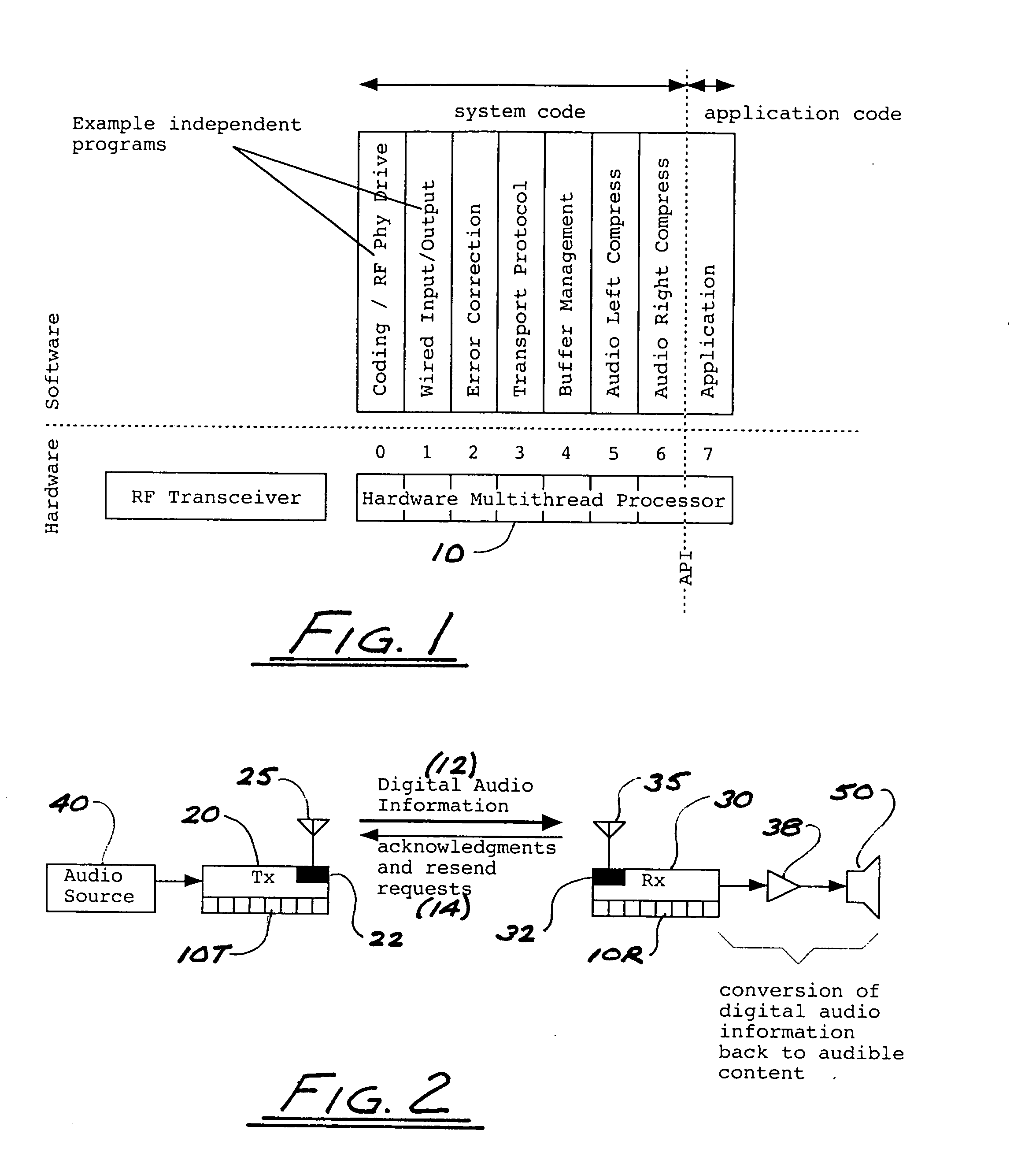
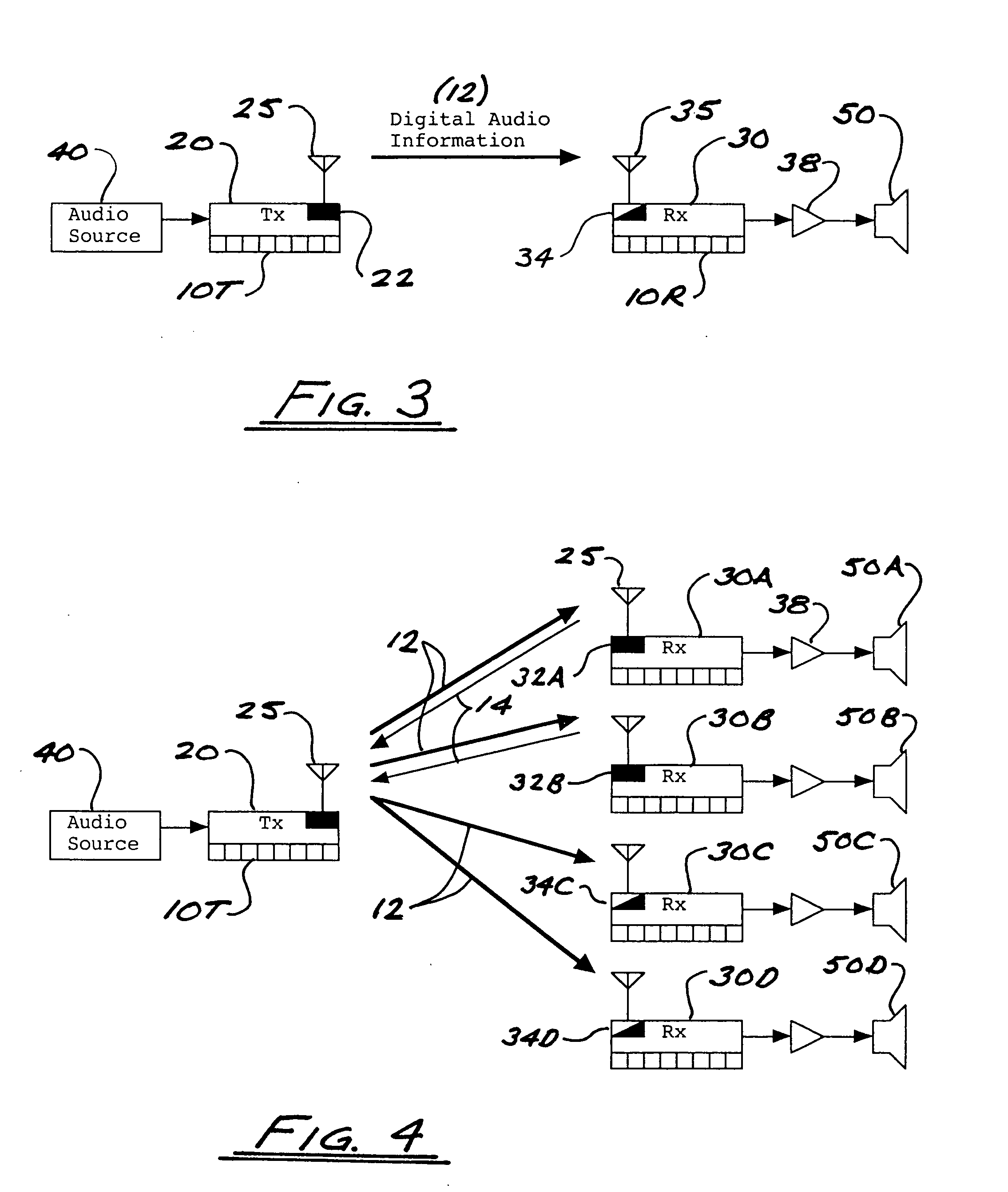
ActiveUS8050203B2Improve service qualityReduce dataError preventionStereophonic circuit arrangementsComputer hardwareDigital radio
In a multi-channel digital wireless audio system with at least one transmit node and at least one receive node, each node can both receive and transmit digital audio signals. Signals sent from a receive node to a transmit node may acknowledge satisfactory signal receipt, or may requesting retransmission of data packets received in a corrupted state. Original and retransmitted signals may be sent in compressed form to enable use of narrow-band digital radios. The system preferably incorporates a dual control channel to enable transmission of meta data. Each system node preferably incorporates a hardware-multithreaded processor adapted to implement various functions such as baseband functions, RF protocol functions, error correction functions, and audio processing functions, with each independent thread being adapted to implement a different functional block.
Owner:ELEVEN ENG
System and method for the detection and termination of fraudulent services
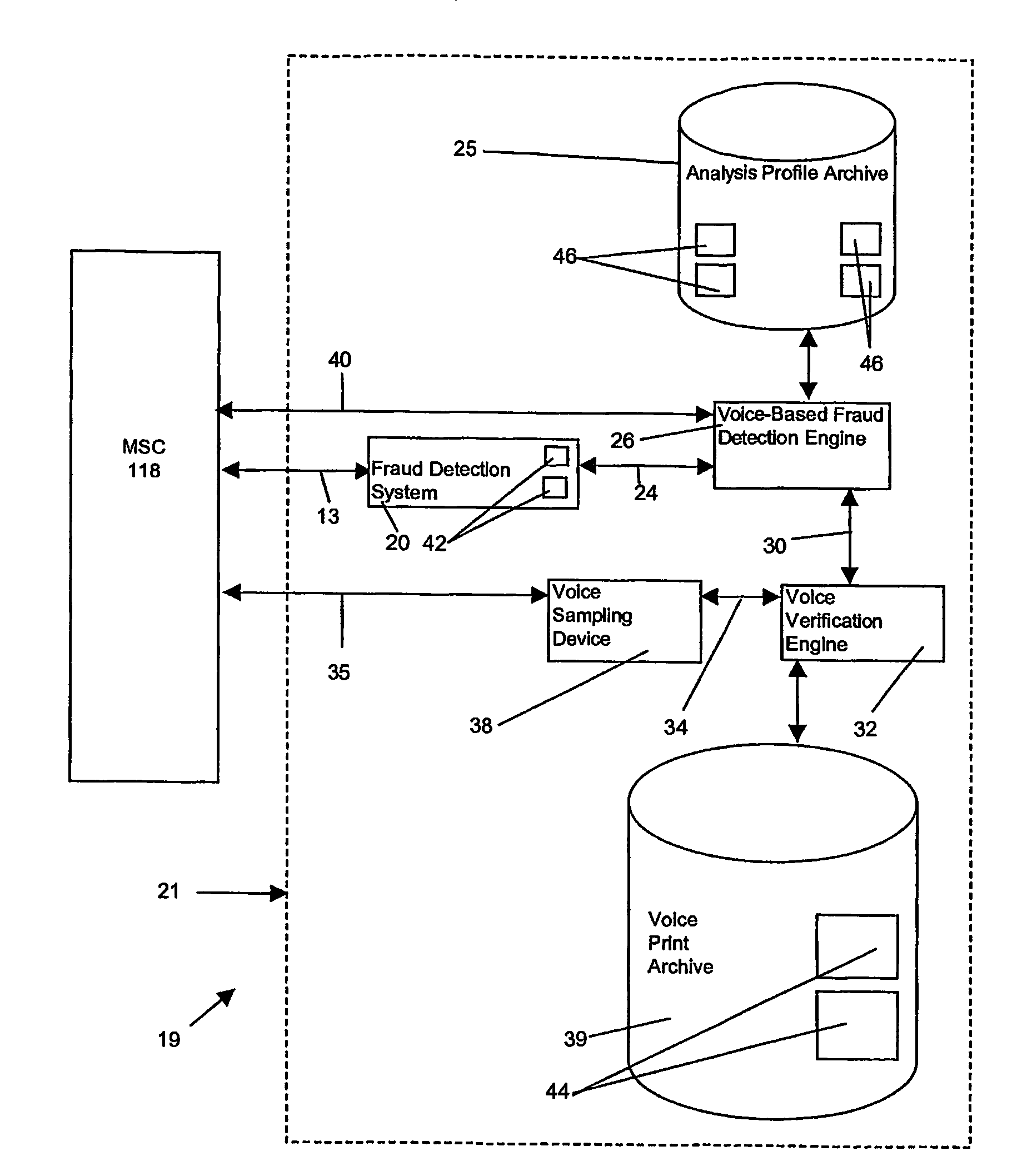
InactiveUS7512221B2High error rateMaximize throughputUnauthorised/fraudulent call preventionEavesdropping prevention circuitsComputer networkFinancial transaction
A system is disclosed for the automatic detection of fraudulent activity on a transaction network, for which each transaction over the network has an associated identifier. In one embodiment, the system includes voice comparison means for comparing a first sampled voice of a user of a first transaction with a subsequently sampled voice of a user of a subsequent transaction having an identical identifier to that of the first transaction. Control means in the form of a voice-based fraud detection engine is provided for determining, from said comparison, a profile of user usage that is representative of a total number of different users of the associated identifier. In a preferred embodiment, the system also includes voice sampling means for sampling a voice of the user of the first transaction to generate a first voice sample.
Owner:CEREBRUS SOLUTIONS LTD
Extended interior methods and systems for spectral, optical, and photoacoustic imaging
ActiveUS20110282181A1Reduce discrepancyMinimize variationReconstruction from projectionRadiation/particle handlingTomographyComputed tomography
The present invention relates to the field of medical imaging. More particularly, embodiments of the invention relate to methods, systems, and devices for imaging, including for tomography-based applications. Embodiments of the invention include, for example, a computed tomography based imaging system comprising: (a) at least one wide-beam gray-scale imaging chain capable of performing a global scan of an object and acquiring projection data relating to the object; (b) at least one narrow-beam true-color imaging chain capable of performing a spectral interior scan of a region of interest (ROI) of and acquiring projection data relating to the object; (c) a processing module operably configured for: (1) receiving the projection data; (2) reconstructing the ROI into an image by analyzing the data with a color interior tomography algorithm, aided by an individualized gray-scale reconstruction of an entire field of view (FOV), including the ROI; and (d) a processor for executing the processing module. The extended interior methods and systems for spectral, optical, and photoacoustic imaging presented in this application can lead to better medical diagnoses by providing images with higher resolution or quality, and can lead to safer procedures by providing systems capable of reducing a patient's exposure time to, and thus quantity of, potentially harmful x-rays. Embodiments of the invention also provide tools for real-time tomography-based analyses.
Owner:VIRGINIA TECH INTPROP INC
Apparatus and method for real-time mining and reduction of streamed data
InactiveUS20070078802A1Reduce the amount requiredReduce data volumeDigital data information retrievalMultiple digital computer combinationsStreaming dataDatabase
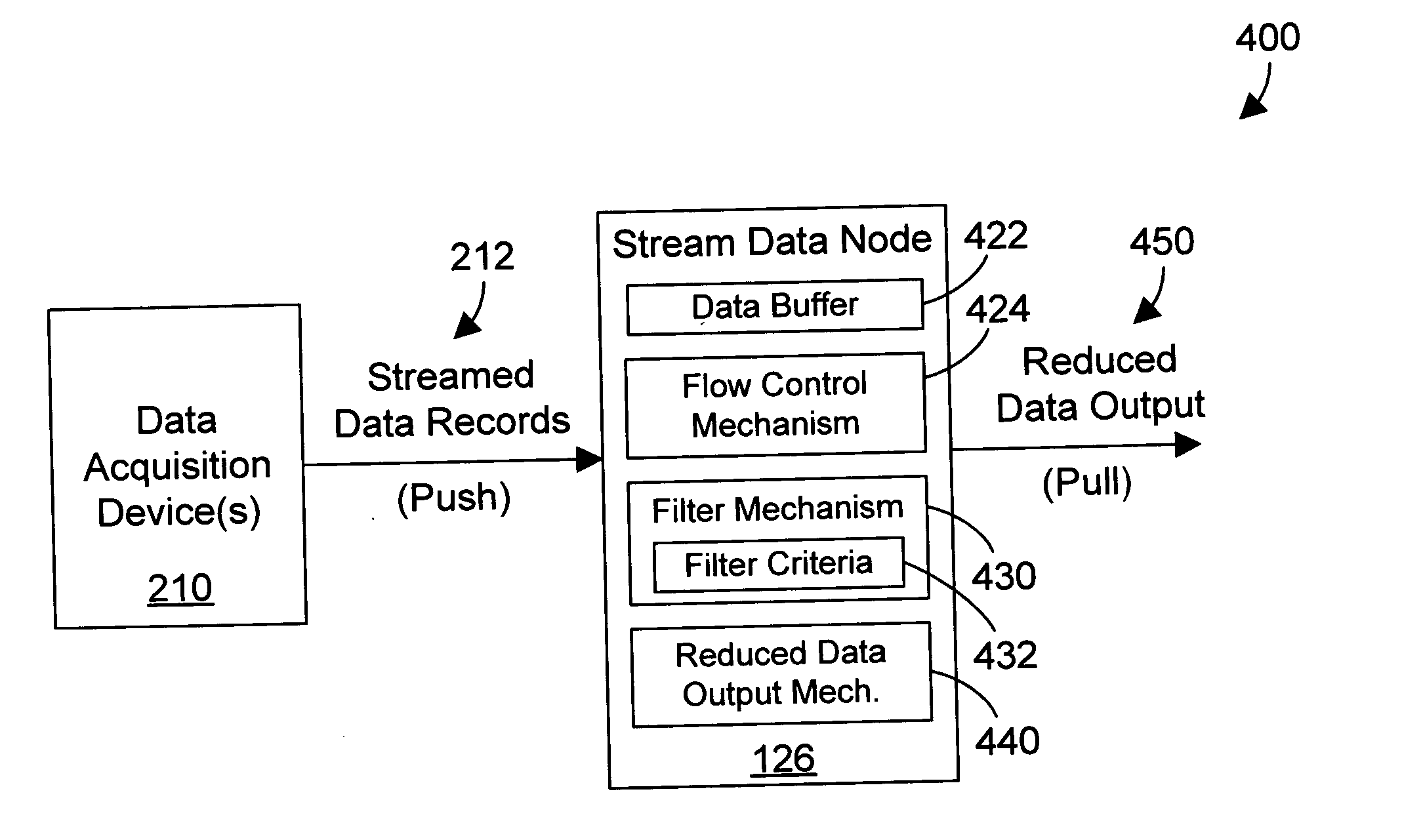
A stream data node receives real-time streamed data from one or more input devices, dynamically filters the streamed data to reduce the streamed data, and delivers the reduced data when requested. By providing real-time filtering of the data, the amount of data that must be stored in a database may be substantially reduced. The stream data node can perform aggregation functions, group functions, and select functions, thereby also significantly reducing the amount of data that must be stored in a database. The stream data node may also be part of a query execution data structure, where it delivers its data when requested by another node in the query execution data structure.
Owner:IBM CORP
Systems and methods for endoscopic angle-resolved low coherence interferometry
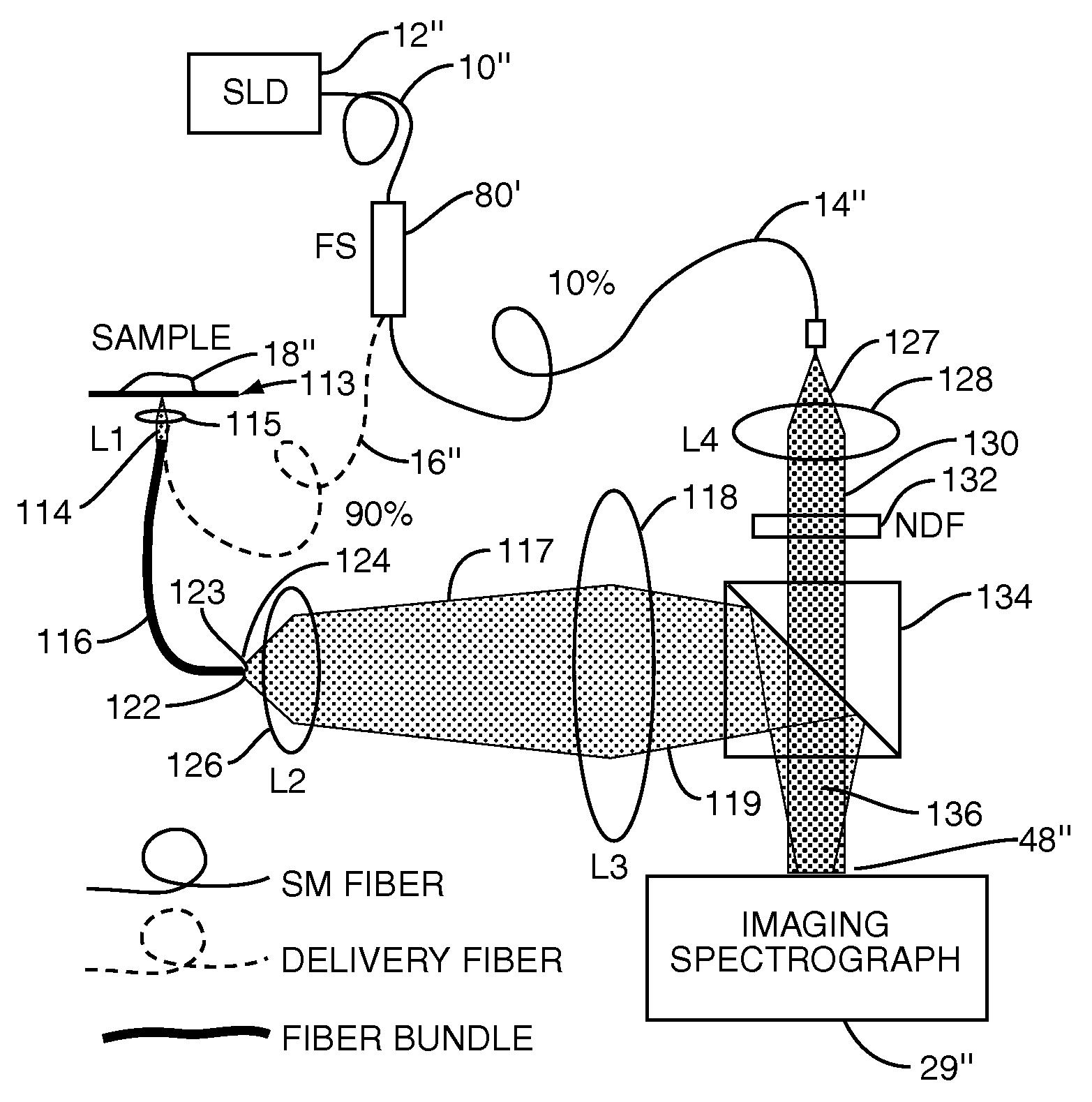
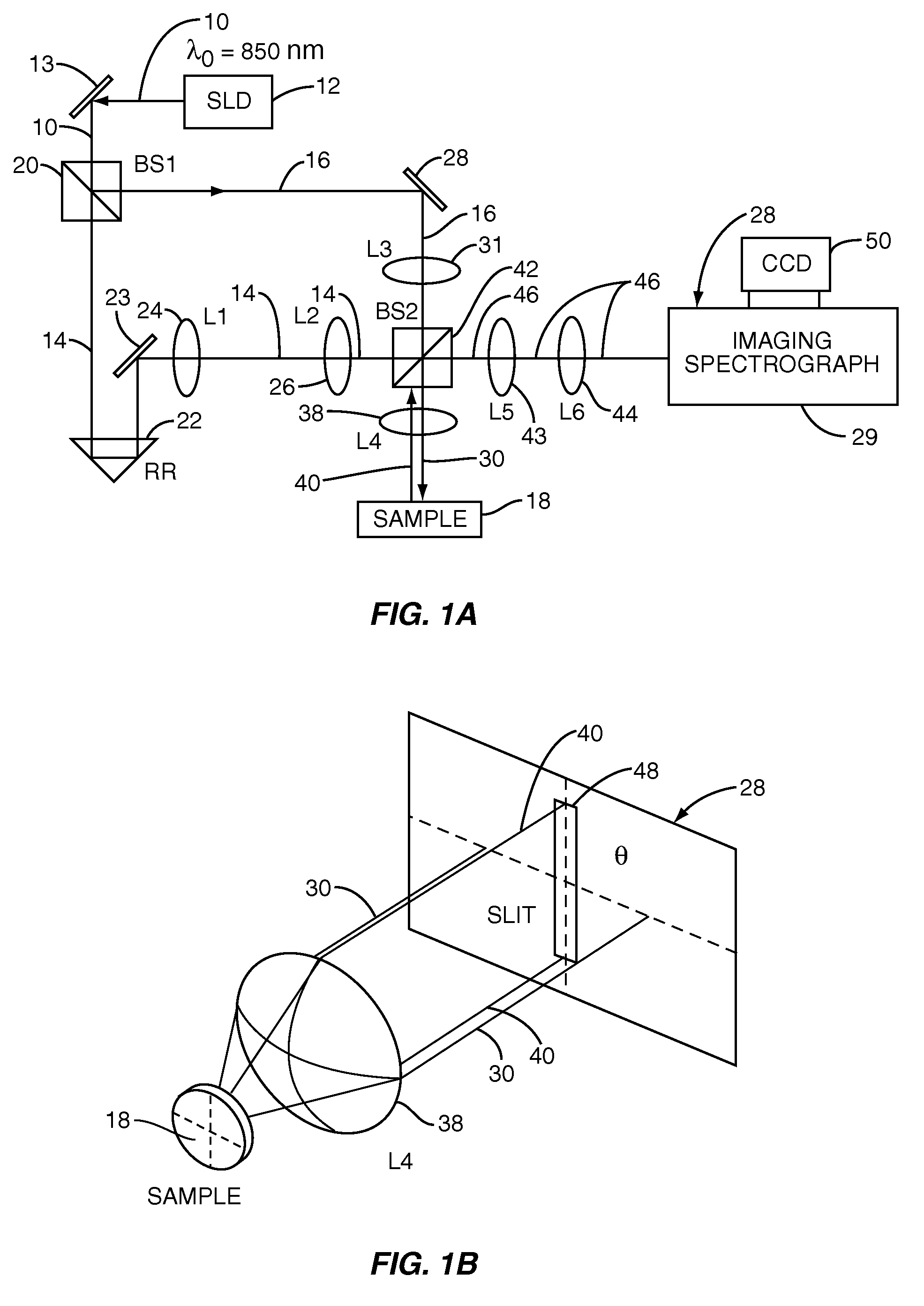
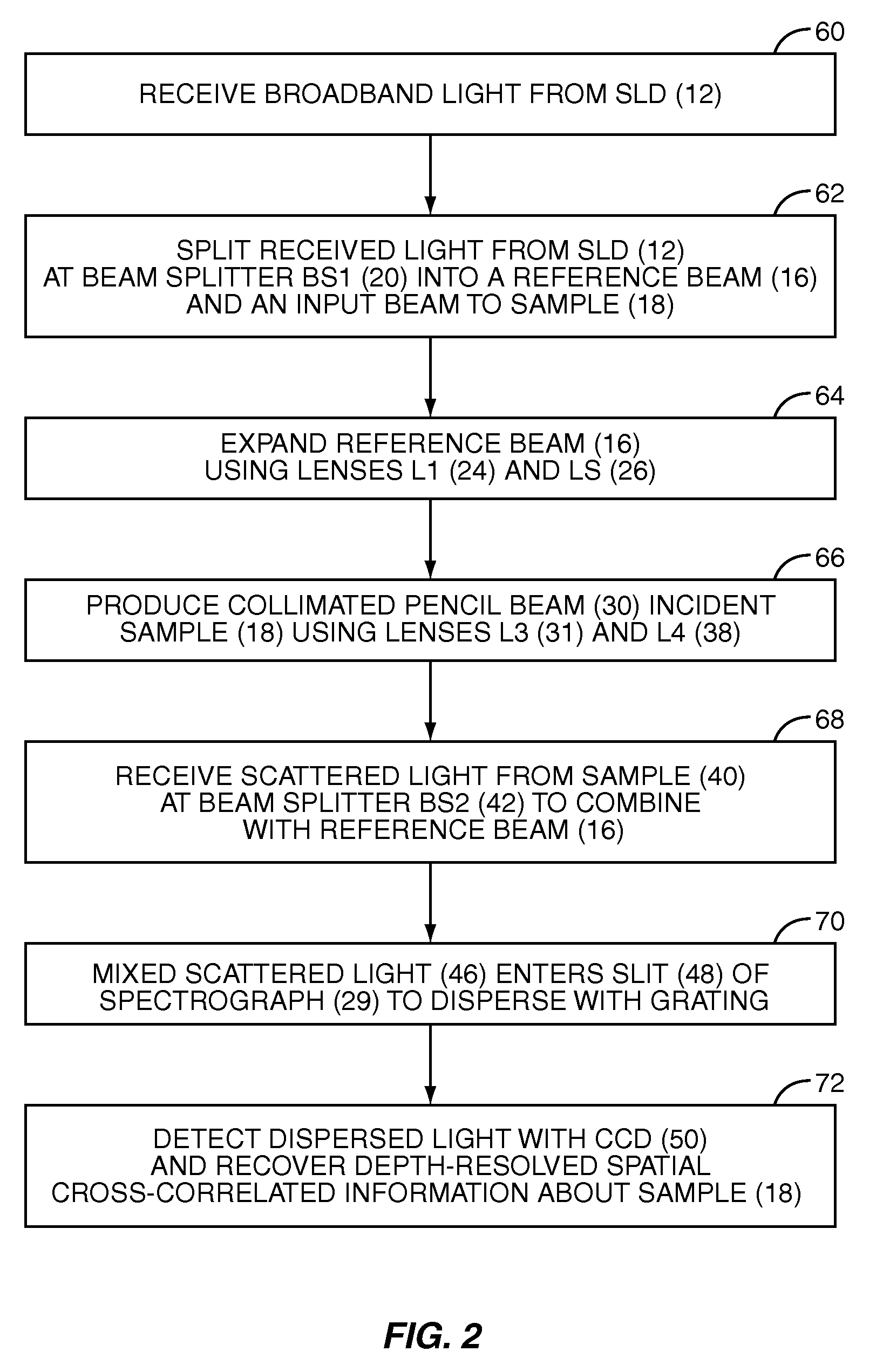
InactiveUS20070133002A1Fast resultsEnhance its widespread applicabilityRadiation pyrometryRaman/scattering spectroscopyData acquisitionIn vivo
Fourier domain a / LCI (faLCI) system and method which enables in vivo data acquisition at rapid rates using a single scan. Angle-resolved and depth-resolved spectra information is obtained with one scan. The reference arm can remain fixed with respect to the sample due to only one scan required. A reference signal and a reflected sample signal are cross-correlated and dispersed at a multitude of reflected angles off of the sample, thereby representing reflections from a multitude of points on the sample at the same time in parallel. Information about all depths of the sample at each of the multitude of different points on the sample can be obtained with one scan on the order of approximately 40 milliseconds. From the spatial, cross-correlated reference signal, structural (size) information can also be obtained using techniques that allow size information of scatterers to be obtained from angle-resolved data.
Owner:DUKE UNIV
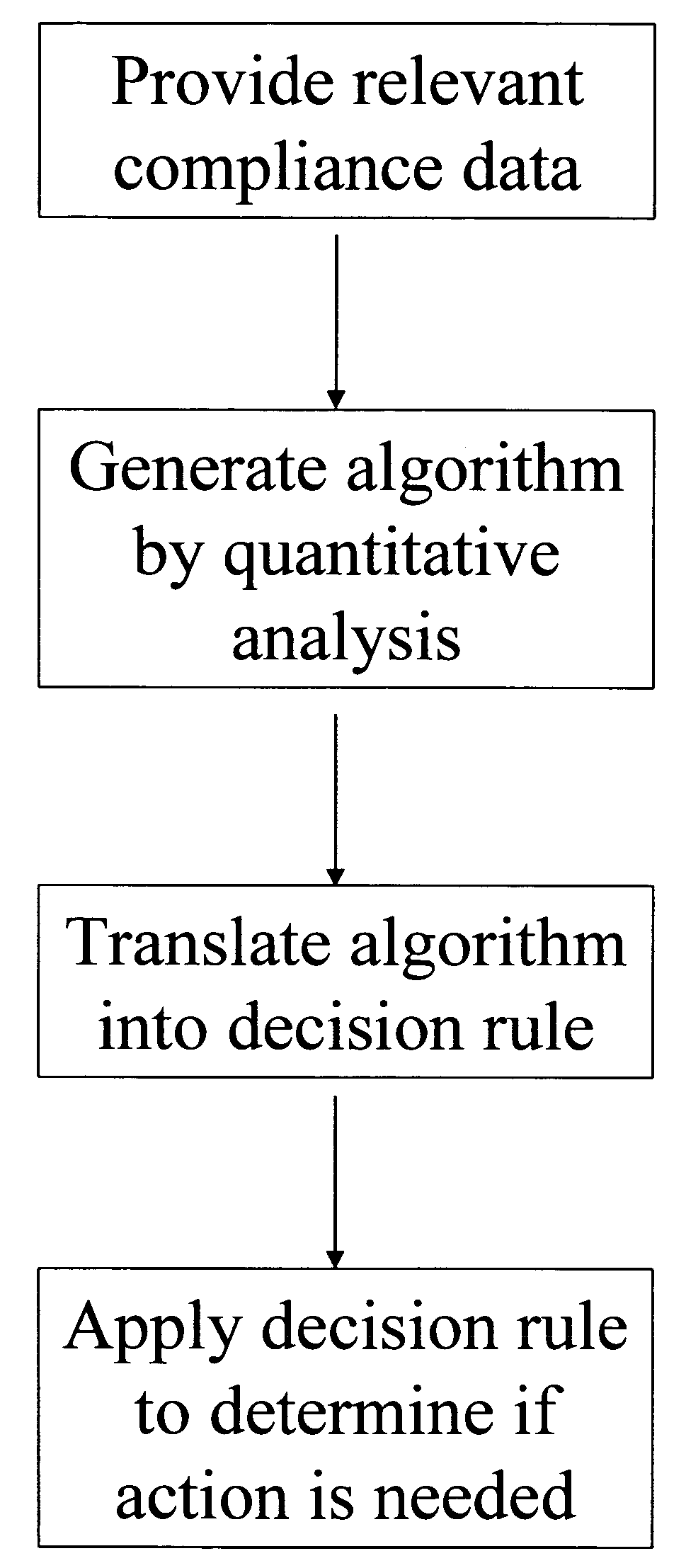
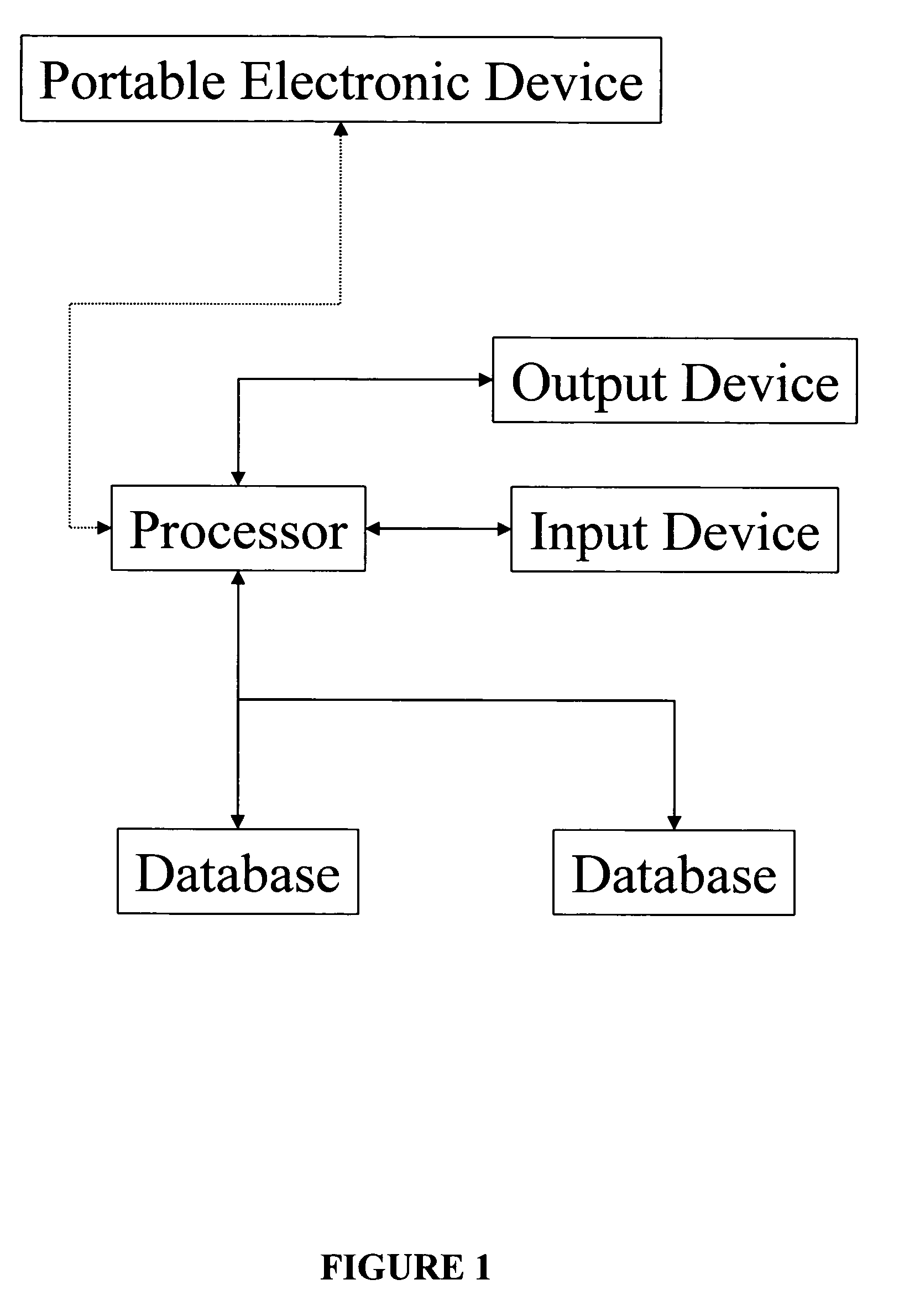
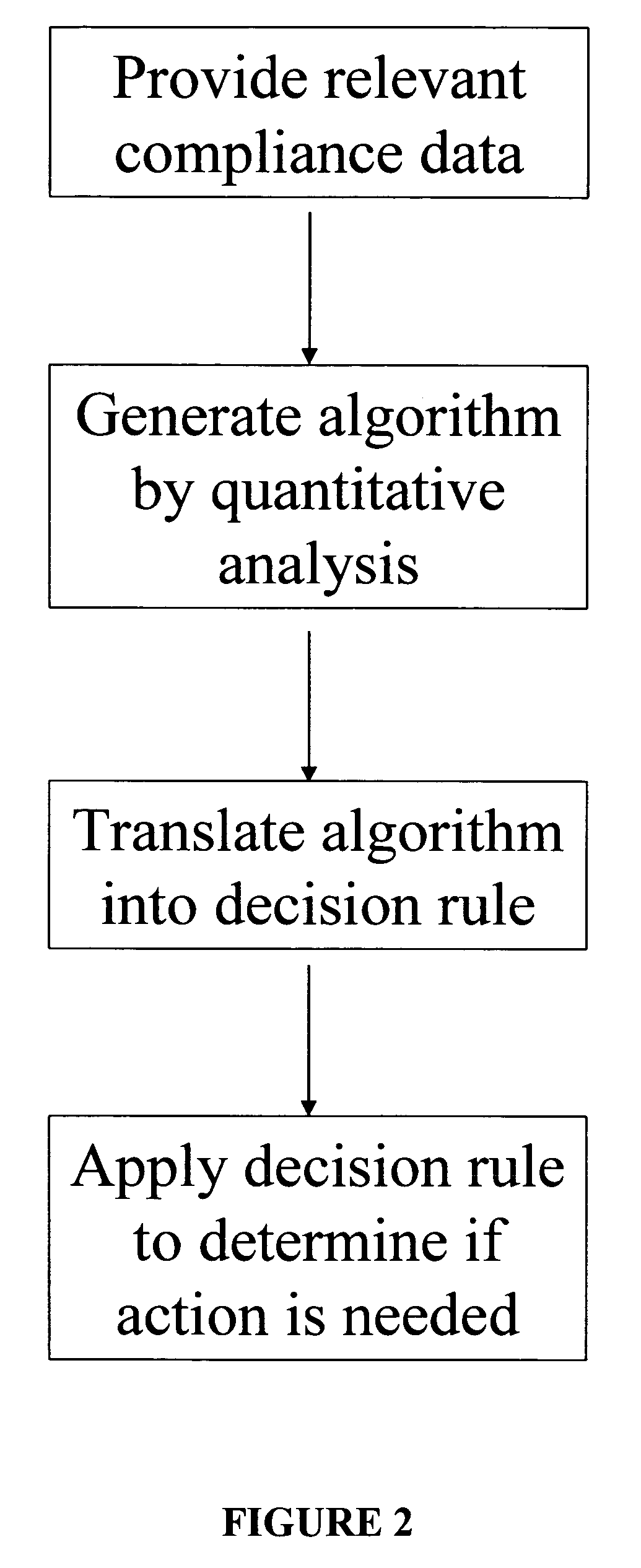
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS7415447B2Reduce testing costsImprove statistics performanceDigital computer detailsForecastingNon complianceClinical trial
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
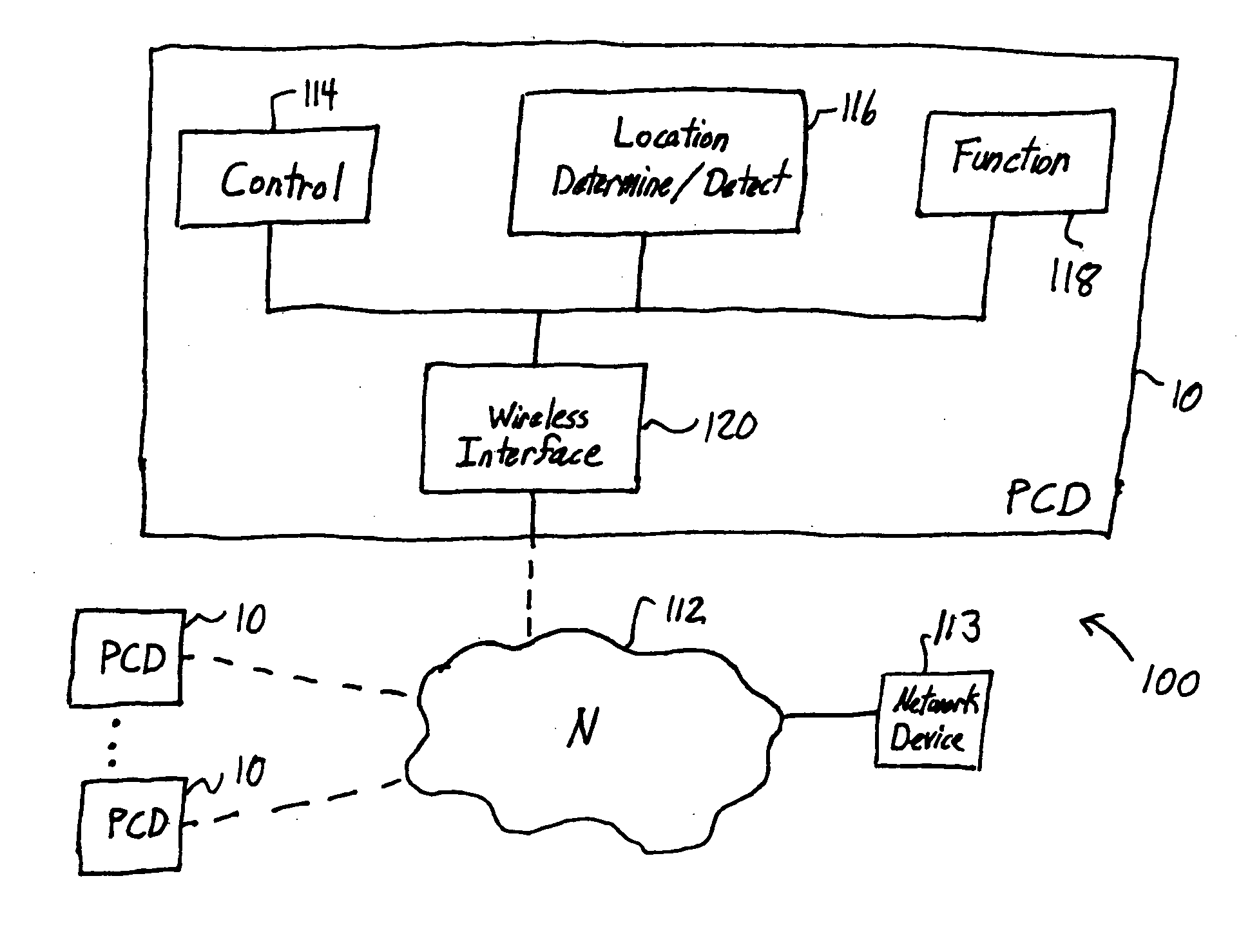
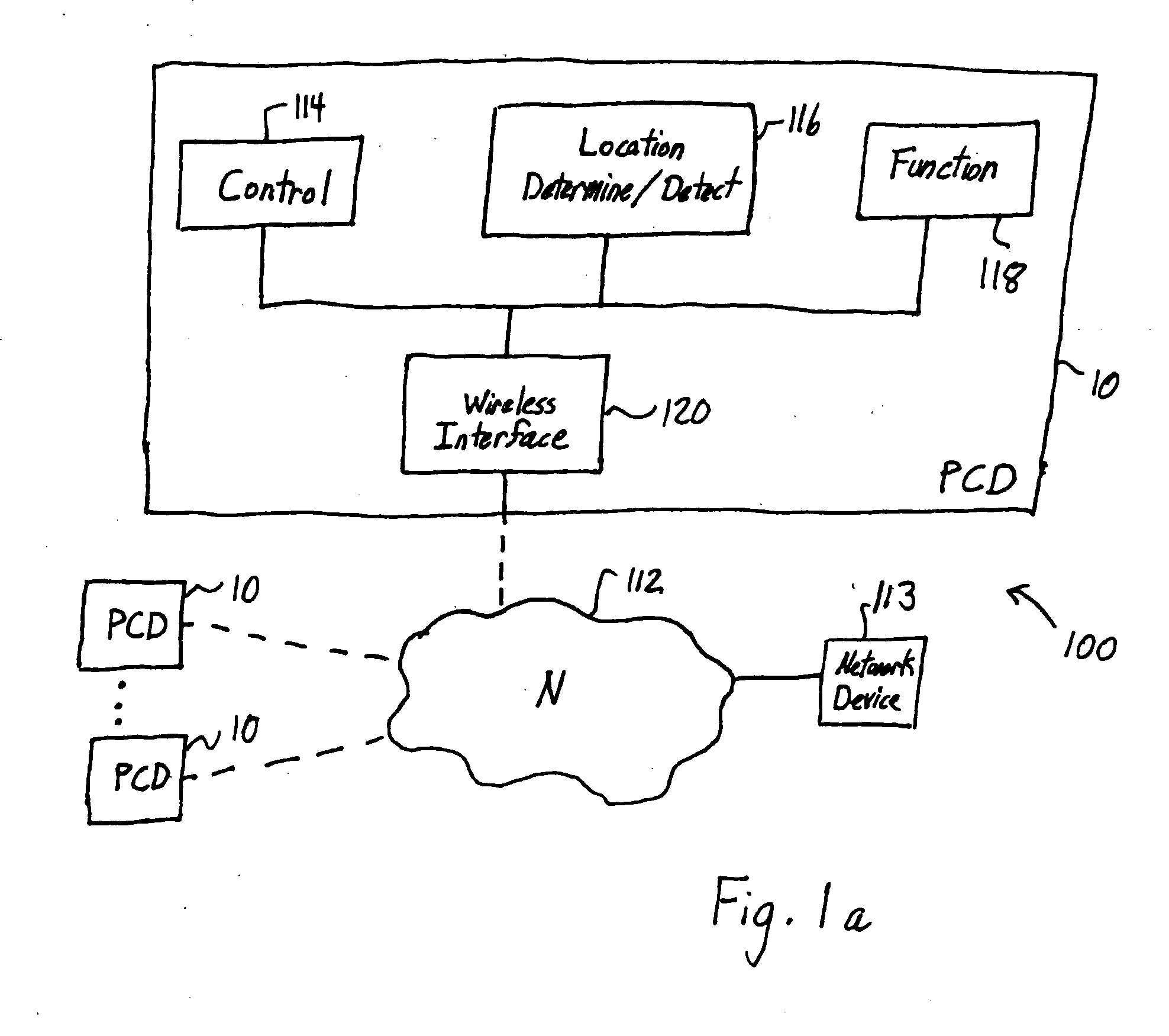
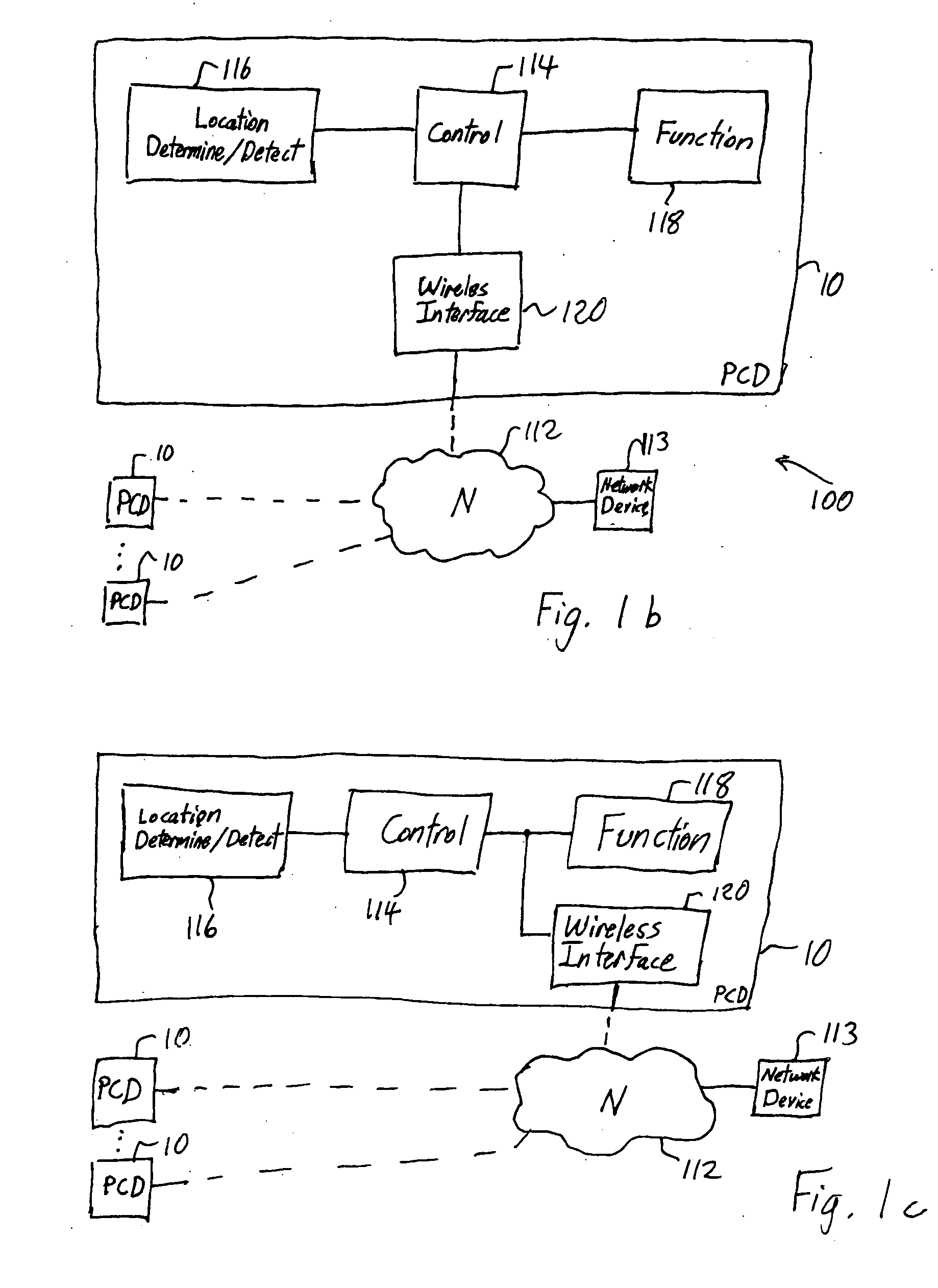
Location-based control of functions of electronic devices
InactiveUS20050221841A1Reduce and eliminate commercial valueResolution of data is reducedAssess restrictionRadio/inductive link selection arrangementsGeographic regionsData stream
A mobile personal communication device, such as a mobile telephone (cellular, satellite, or the like), that is capable of determining its geographic location, through an on-board GPS unit or other geographic locating mechanism, has a function that is disabled, enabled, modified or otherwise controlled when the personal communication device is determined to be within a defined area. The function controlled based on geographic region may include: the transmission of a data stream, such as a video or audio data stream, from or to the personal communication device; the disamblement or modification of ringers or other mechanism for audible notification of an event, for example, an incoming call or message from an external caller; the disablement or modification of an on-board camera or other on-board recording mechanism; and / or the disablement or modification of screen savers, disabling or modification of display lighting or backlighting or other functions of the personal communication device.
Owner:KOLETRY PROCESSING L L C
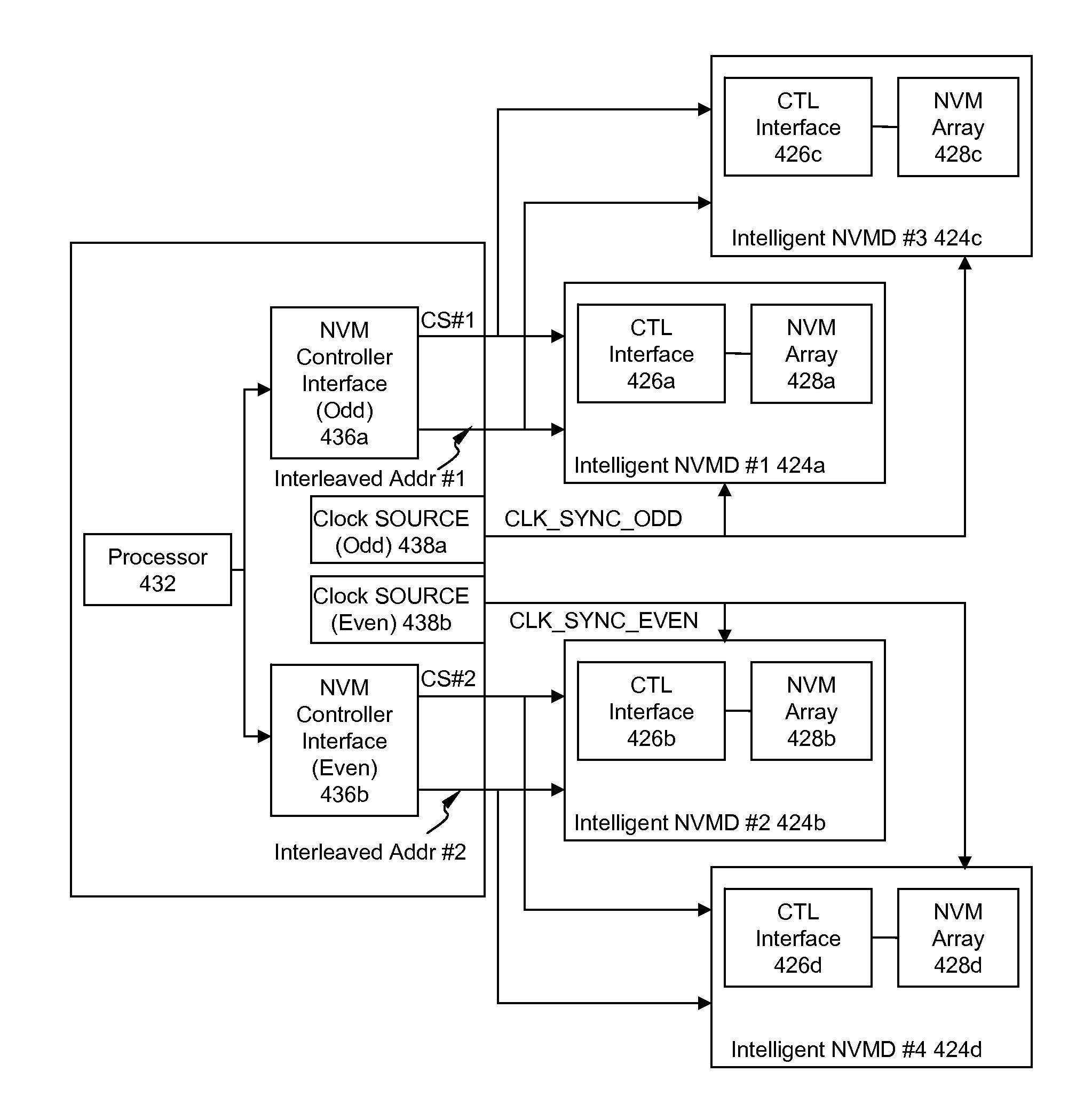
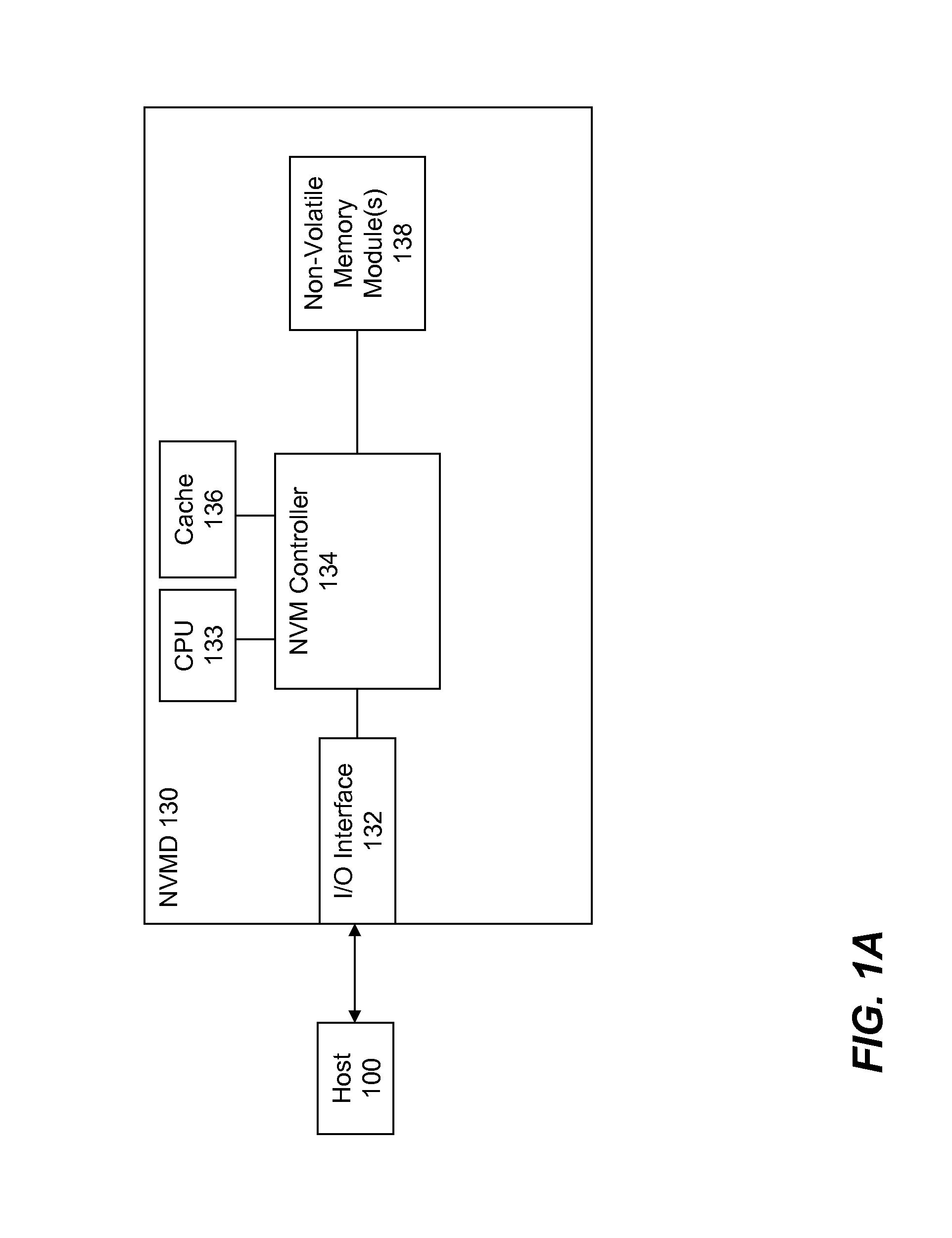
High Performance and Endurance Non-volatile Memory Based Storage Systems
InactiveUS20080320209A1Reduce dataEndurance of the NVM based storage system is increasedMemory architecture accessing/allocationMemory adressing/allocation/relocationPhase locked loop circuitPhysical address
High performance and endurance non-volatile memory (NVM) based storage systems are disclosed. According to one aspect of the present invention, a NVM based storage system comprises at least one intelligent NVM device. Each intelligent NVM device includes a control interface logic and NVM. Logical-to-physical address conversion is performed within the control interface logic, thereby eliminating the need of address conversion in a storage system level controller. In another aspect, a volatile memory buffer together with corresponding volatile memory controller and phase-locked loop circuit is included in a NVM based storage system. The volatile memory buffer is partitioned to two parts: a command queue; and one or more page buffers. The command queue is configured to hold received data transfer commands by the storage protocol interface bridge, while the page buffers are configured to hold data to be transmitted between the host computer and the at least one NVM device.
Owner:SUPER TALENT ELECTRONICS
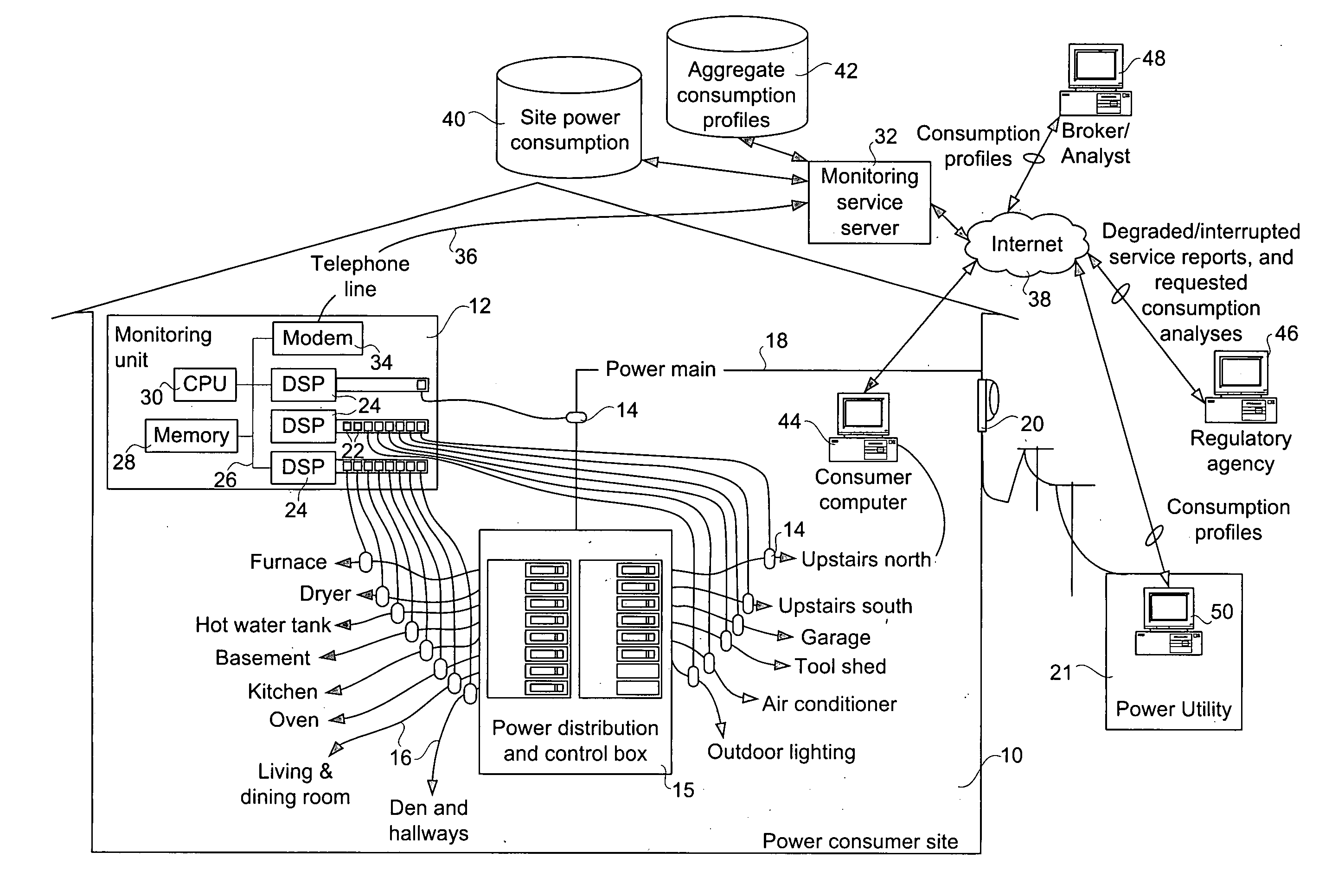
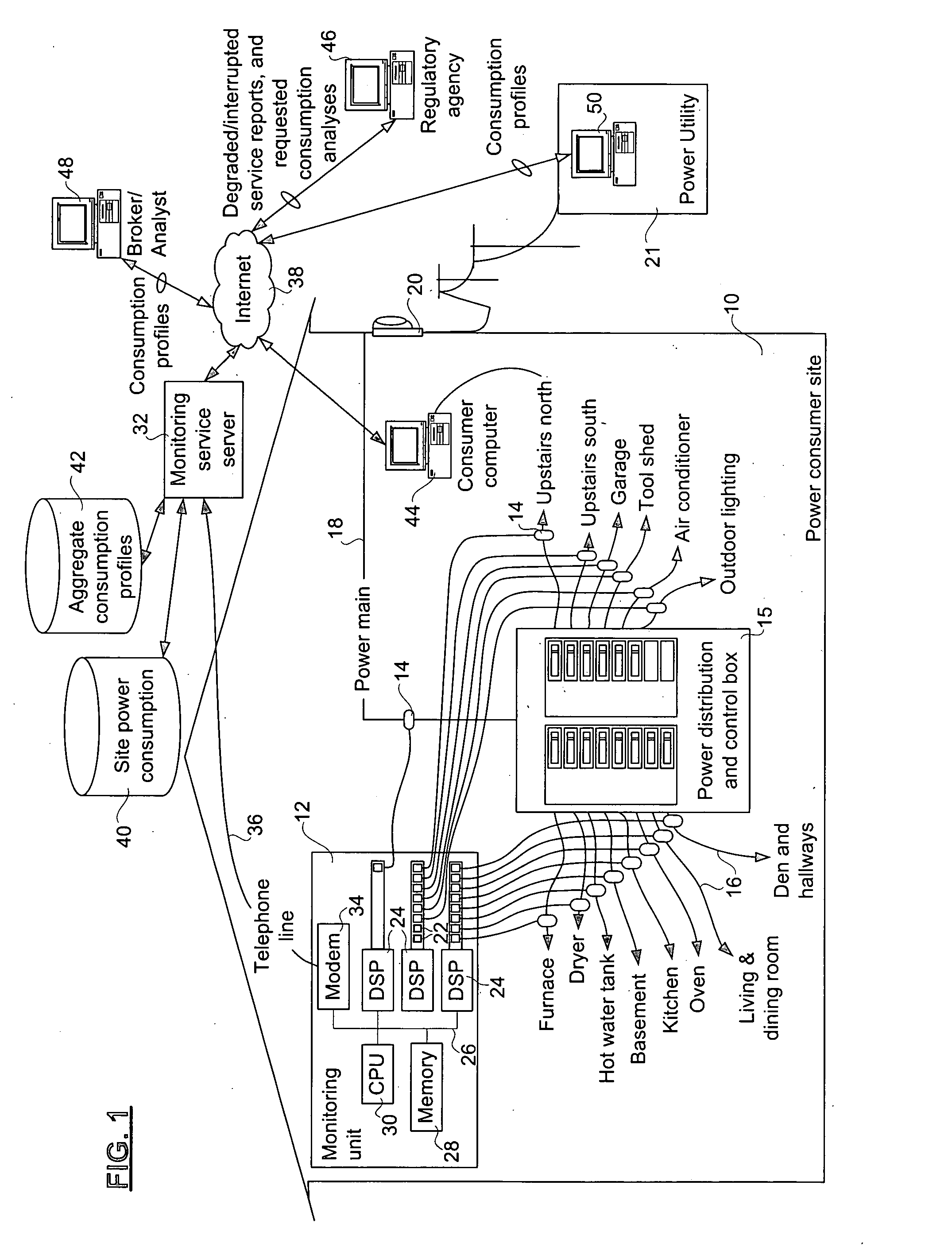
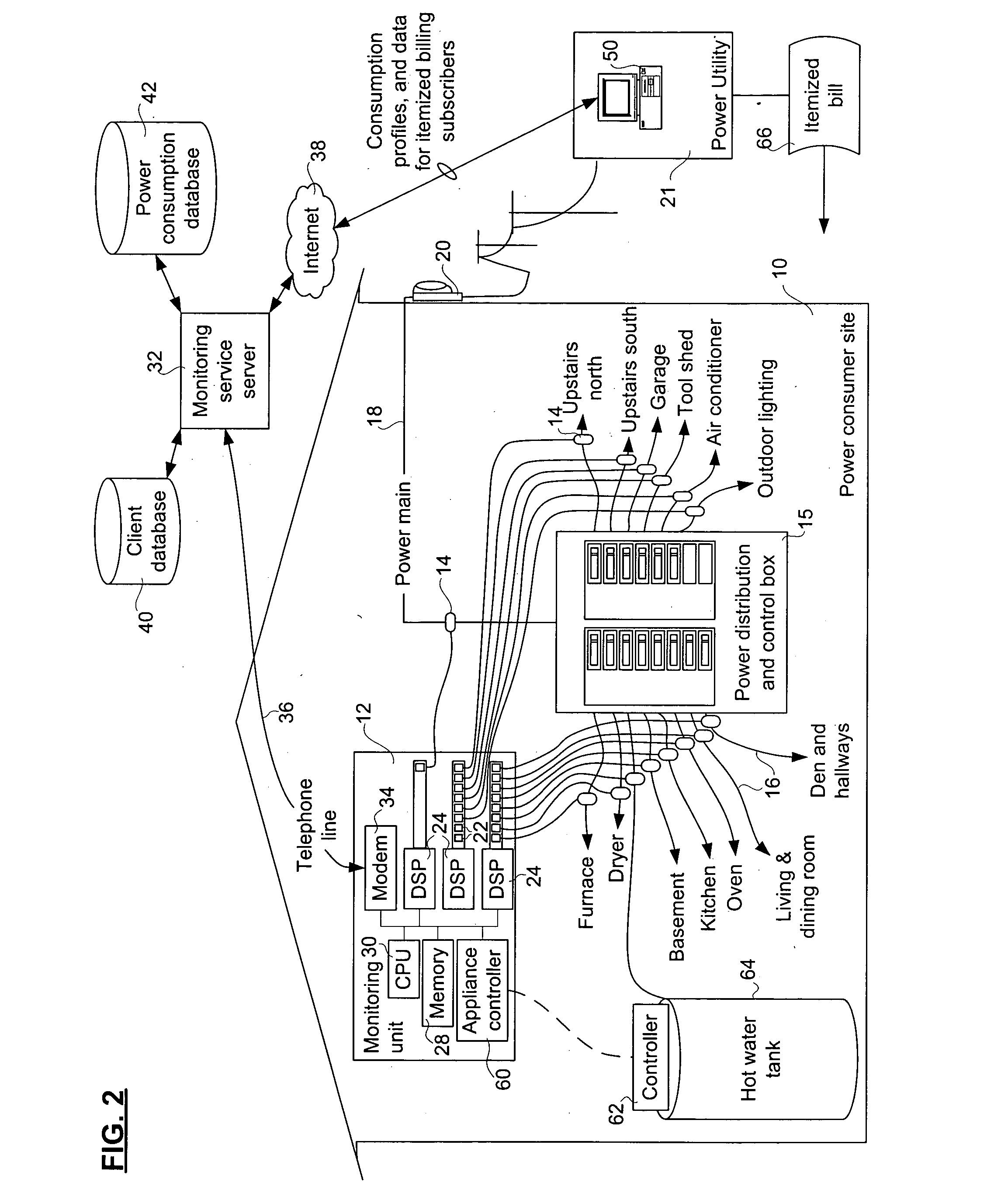
Method and apparatus for monitoring power consumption on power distribution circuits for centralized analysis
InactiveUS20050116836A1Low costReduce dataElectric signal transmission systemsCircuit arrangementsEngineeringElectric power
A method, apparatus, and system for providing a power monitoring service to an electric power consumer at a power consumer site involves providing a monitoring unit at the power consumer site with connections to probes on individual power distribution circuits emanating from a power distribution and control panel of the consumer site. The monitoring unit measures a power consumption of each of the power distribution circuits using the probes, and communicates power consumption data related to the respective power distribution circuits to a power monitoring server that collects and analyzes the power consumption data. The power monitoring server has a circuit description table that identifies each of the power distribution circuits to permit detailed billing to the power consumer. The monitoring server also uses per-circuit information relating to many power consumers to compute at least one aggregate power consumption profile.
Owner:TRIACTA POWER TECH
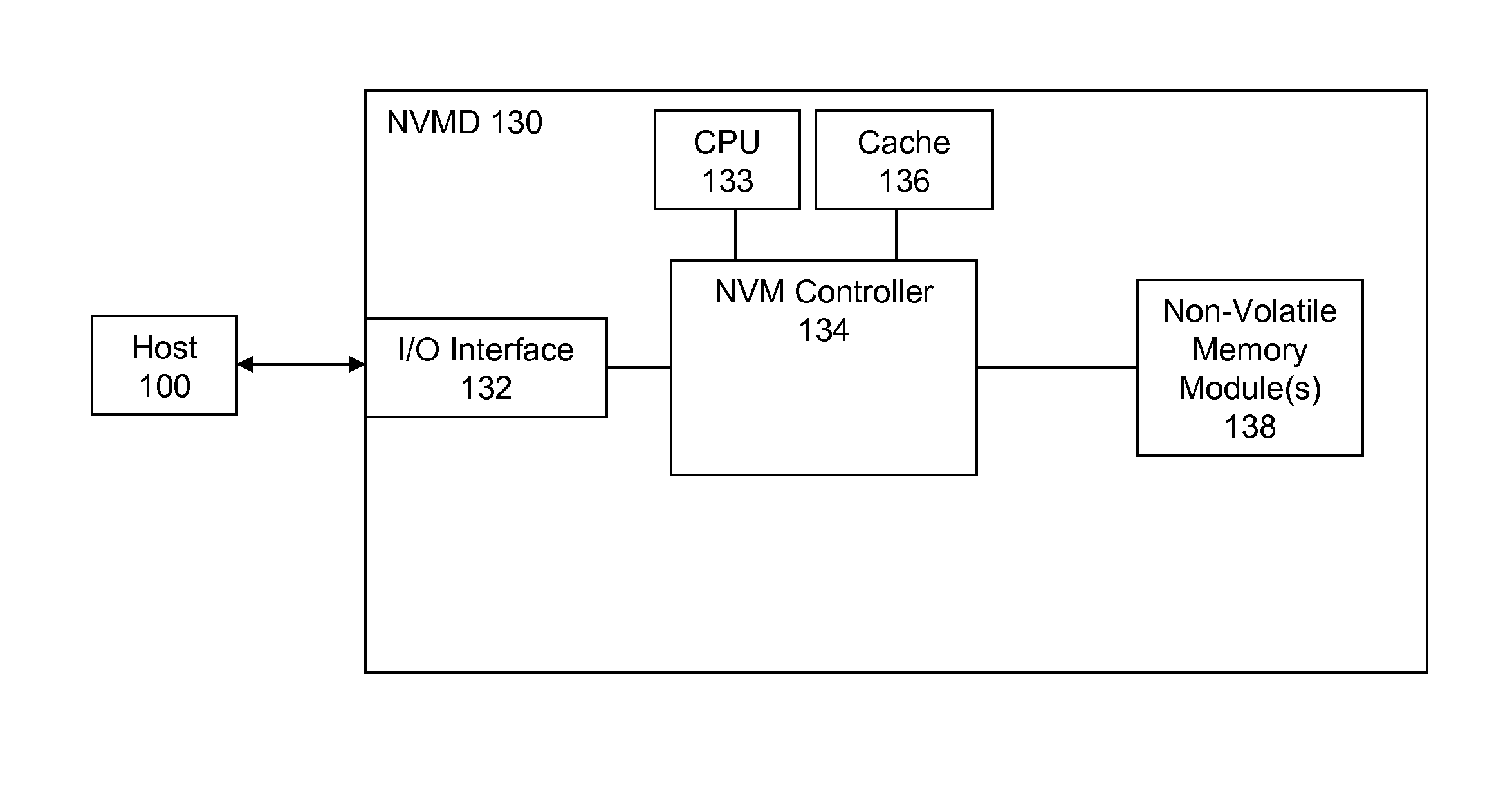
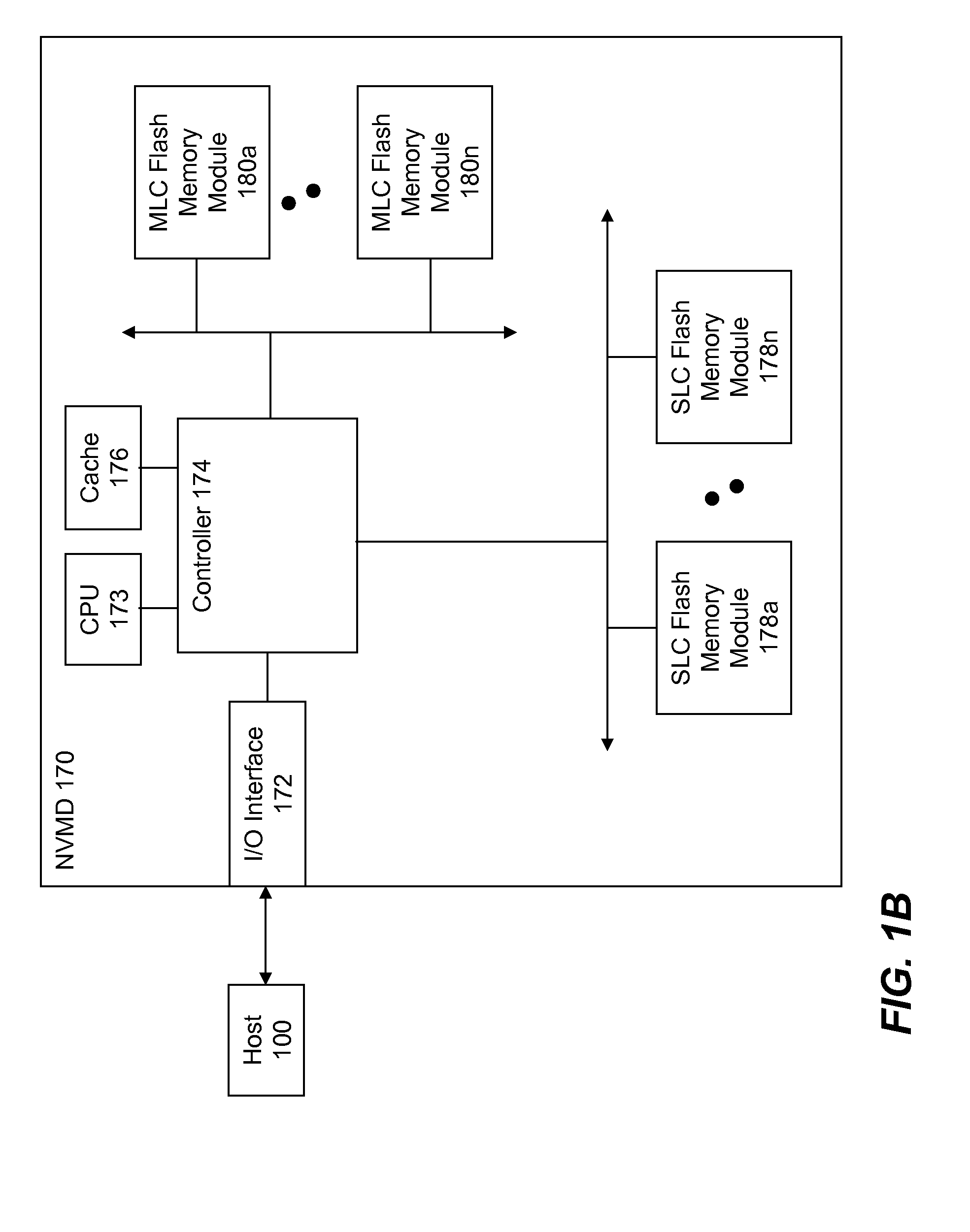
High Endurance Non-Volatile Memory Devices
InactiveUS20080209112A1Reduce data programmingIncreased life-spanMemory architecture accessing/allocationDigital storageData memoryData cache
High endurance non-volatile memory devices (NVMD) are described. A high endurance NVMD includes an I / O interface, a NVM controller, a CPU along with a volatile memory subsystem and at least one non-volatile memory (NVM) module. The volatile memory cache subsystem is configured as a data cache subsystem. The at least one NVM module is configured as a data storage when the NVMD is adapted to a host computer system. The I / O interface is configured to receive incoming data from the host to the data cache subsystem and to send request data from the data cache subsystem to the host. The at least one NVM module may comprise at least first and second types of NVM. The first type comprises SLC flash memory while the second type MLC flash. The first type of NVM is configured as a buffer between the data cache subsystem and the second type of NVM.
Owner:SUPER TALENT TECH CORP
LIDAR Based 3-D Imaging With Varying Illumination Field Density
ActiveUS20170269198A1Reduce energy consumptionReduce consumptionElectromagnetic wave reradiationRadarOperating temperature
Methods and systems for performing three dimensional LIDAR measurements with varying illumination field density are described herein. A LIDAR device includes a plurality of pulse illumination sources and corresponding detectors. The current pulses supplied to the pulse illumination sources are varied to reduce total energy consumption and heat generated by the LIDAR system. In some embodiments, the number of active pulse illumination sources is varied based on the orientation of the LIDAR device, the distance between the LIDAR device and an object detected by the LIDAR device, an indication of an operating temperature of the LIDAR device, or a combination thereof. In some embodiments, the number of active pulse illumination sources is varied based on the presence of an object detected by the LIDAR device or another imaging system.
Owner:VELODYNE LIDAR USA INC
Industry specific brand benchmarking system based on social media strength of a brand
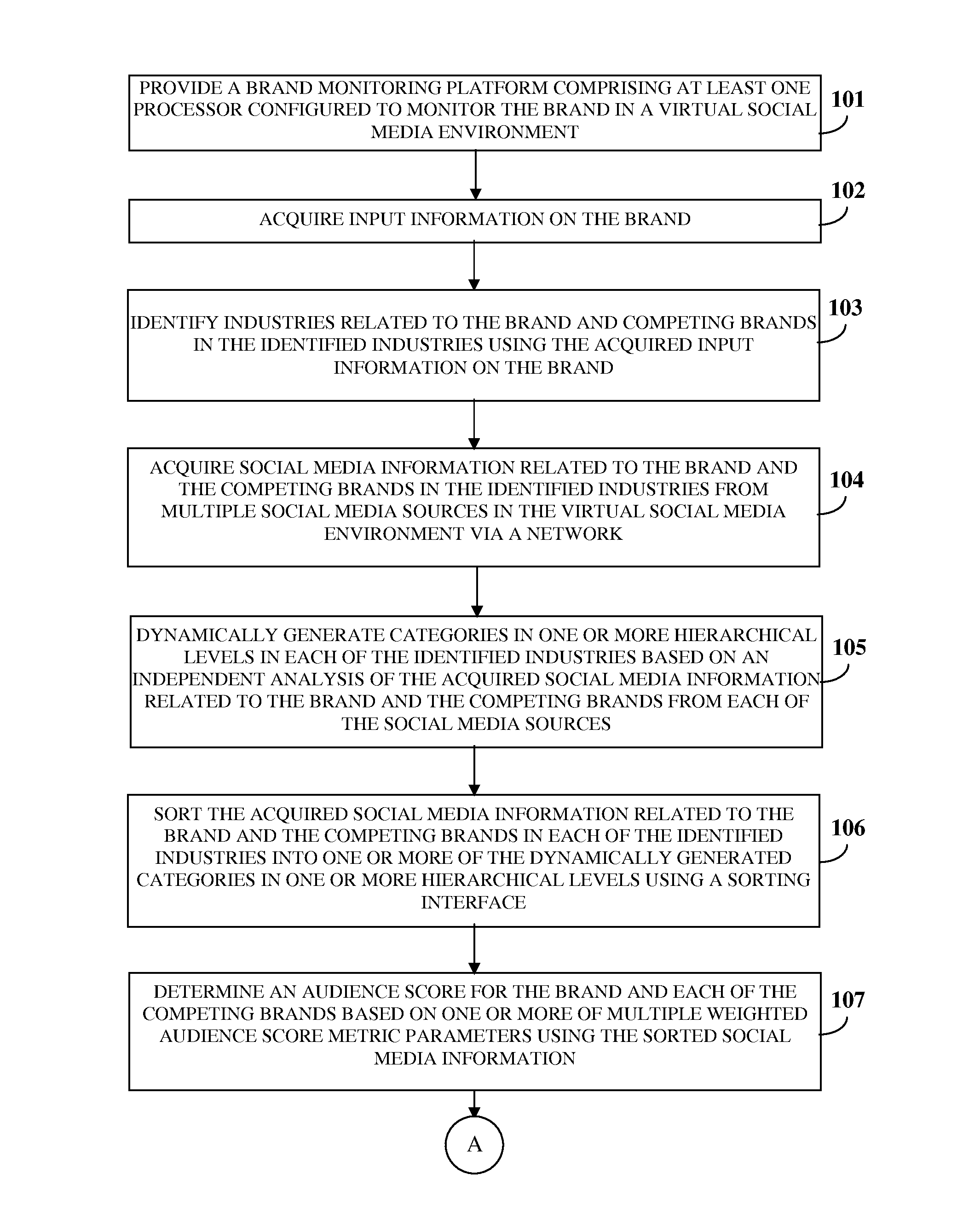
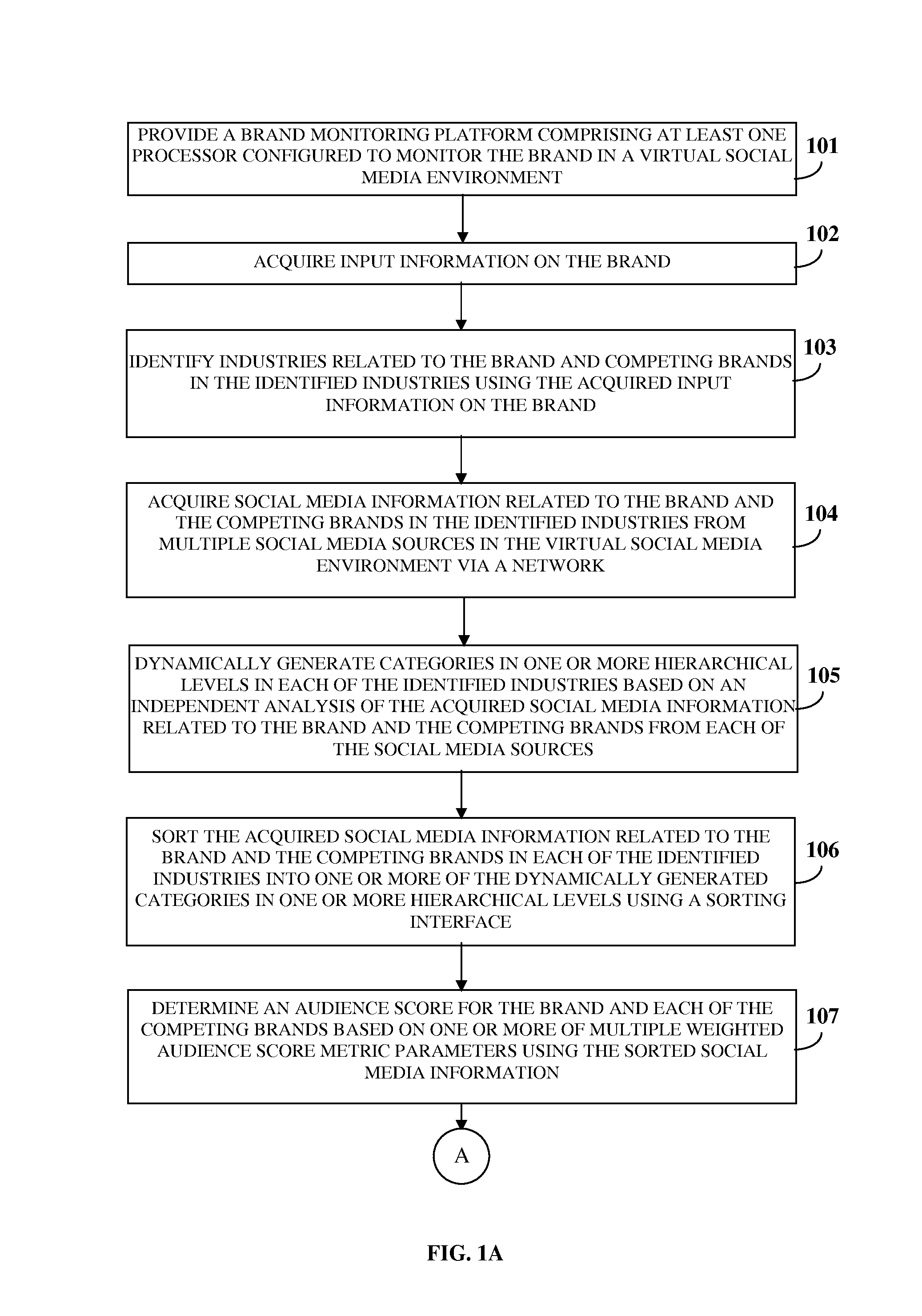
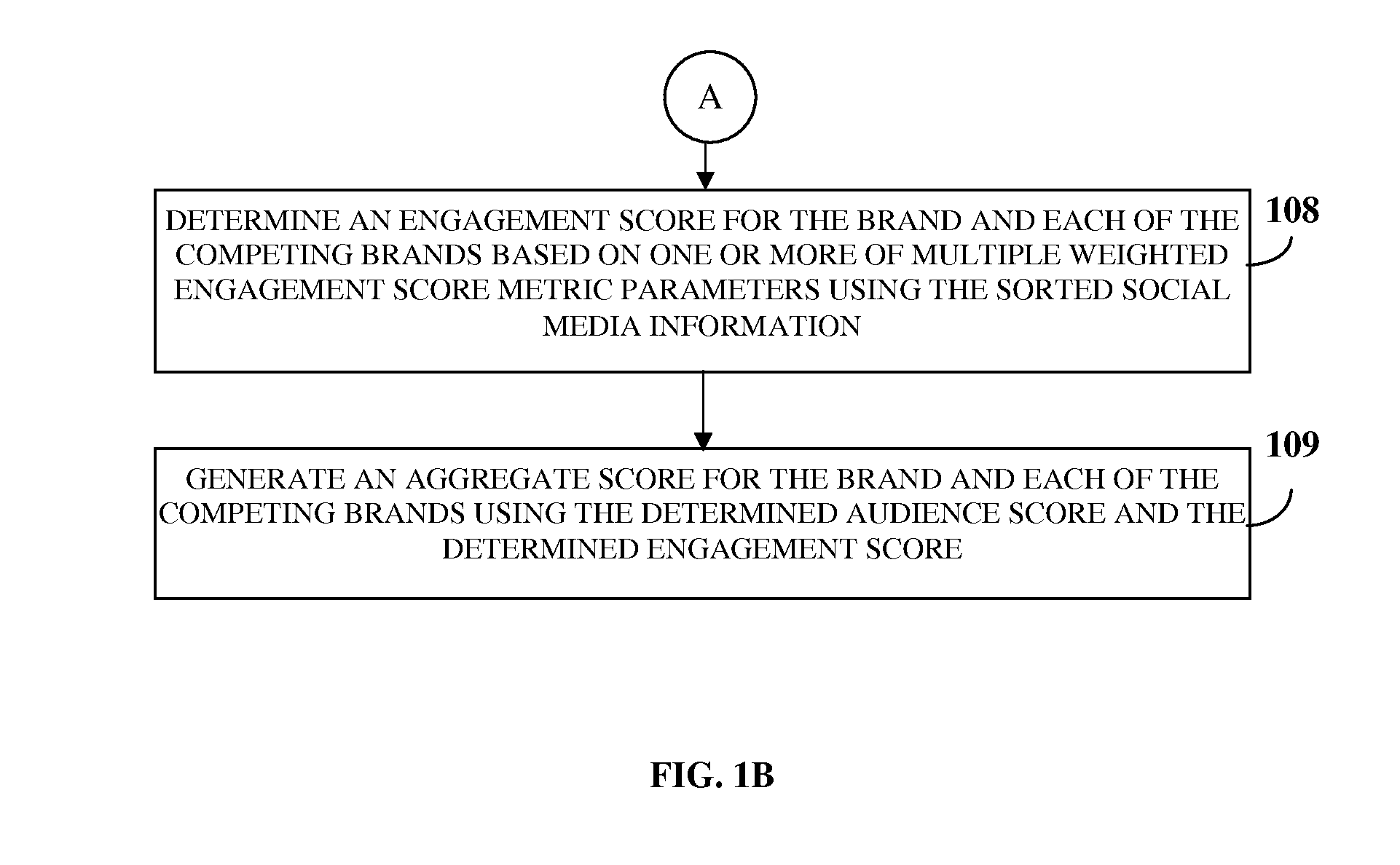
ActiveUS20130325550A1Reduce the differenceReduce dataServices signallingMarketingSocial mediaData science
A brand monitoring platform (BMP) for brand benchmarking based on a brand's social media strength is provided. The BMP acquires input information on the brand and identifies industries related to the brand and competing brands. The BMP acquires social media information related to the brand and the competing brands from multiple social media sources via a network, dynamically generates categories in one or more hierarchical levels in each of the industries based on an independent analysis of the social media information, and sorts the social media information into the categories using a sorting interface. The BMP generates an aggregate score using an audience score determined by measuring an aggregate reach of the brand and the competing brands based on weighted audience score metric parameters, and an engagement score determined by measuring interaction between the brand and the competing brands and their followers based on weighted engagement score metric parameters.
Owner:UNMETRIC
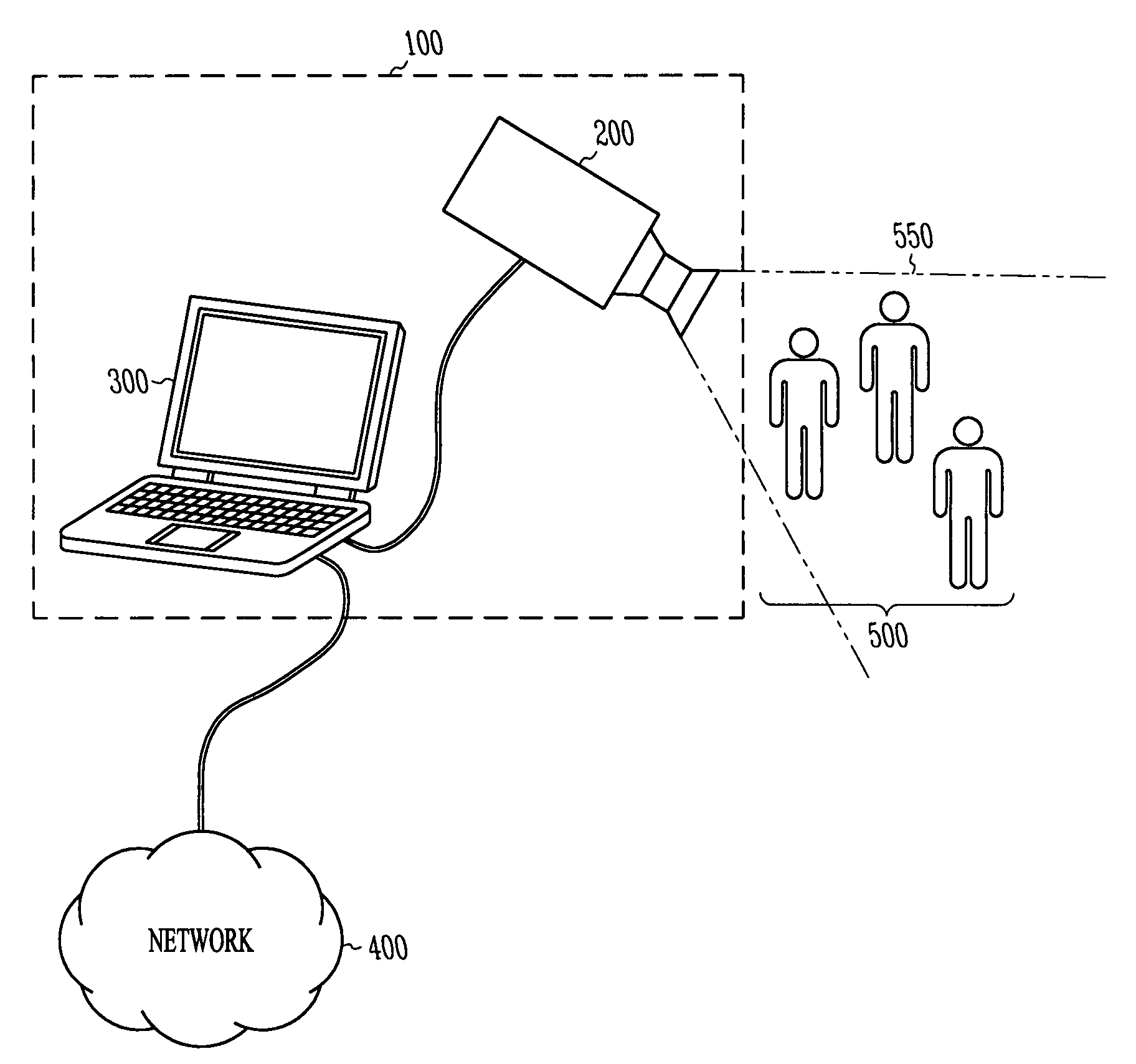
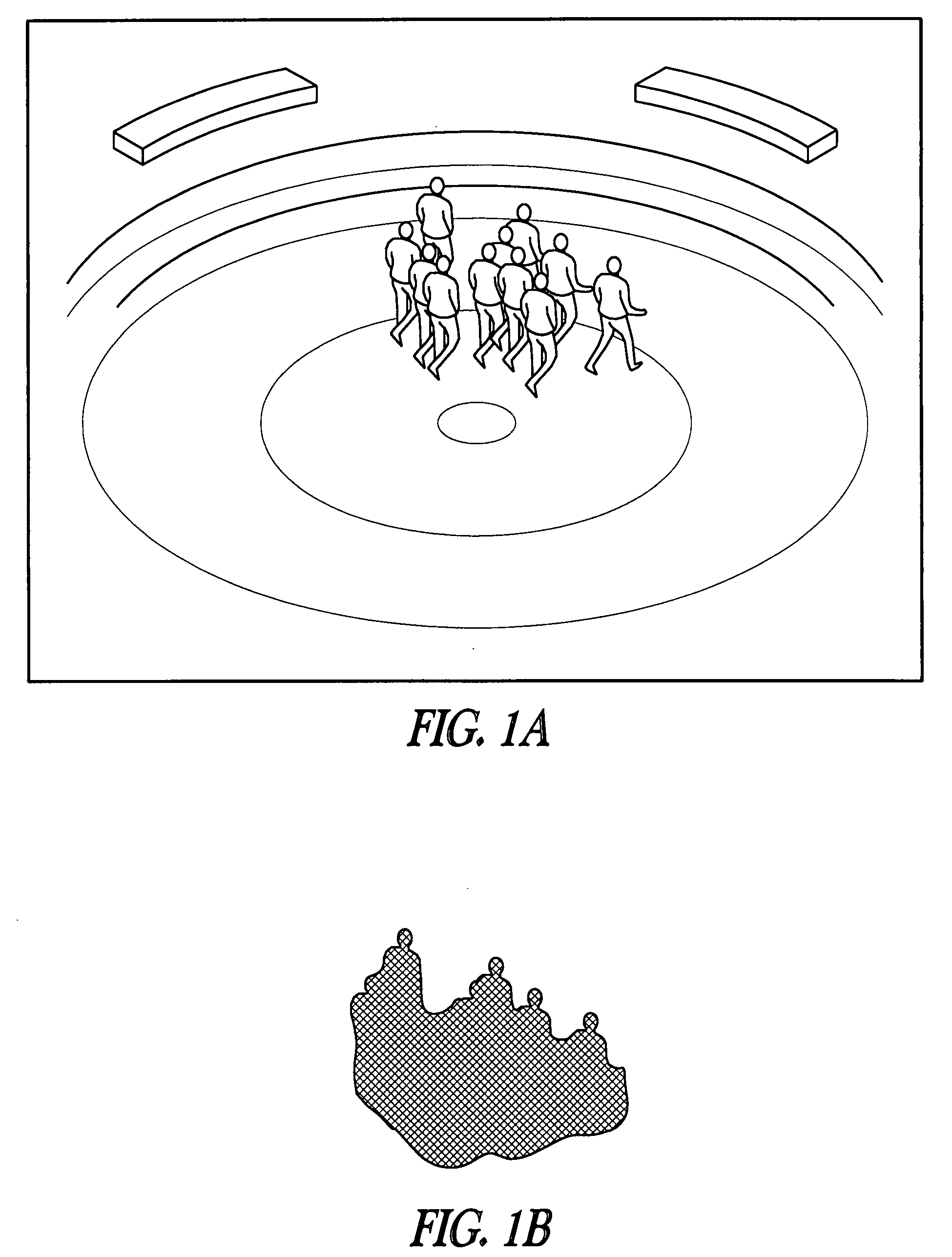
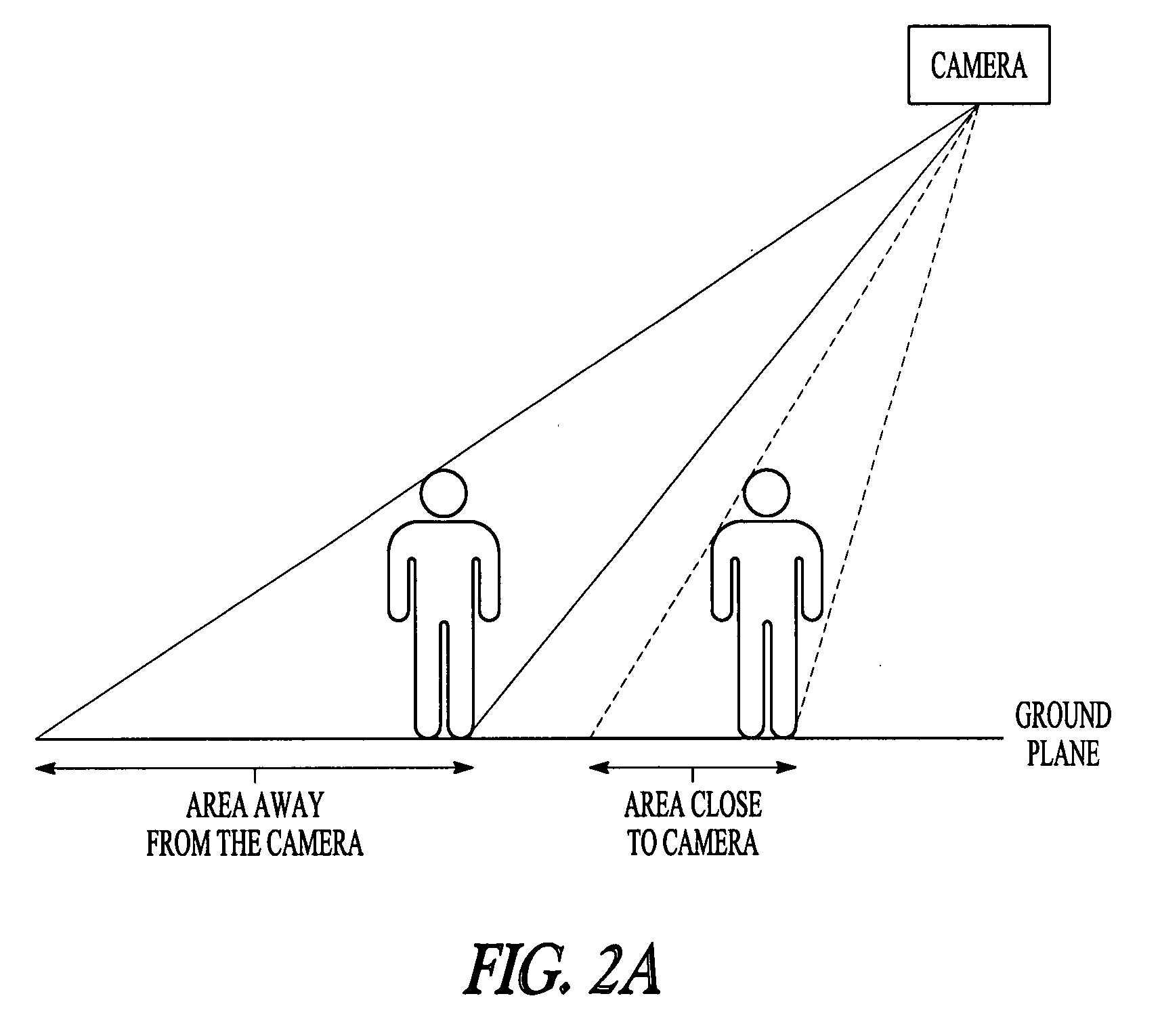
Crowd counting and monitoring
ActiveUS20080118106A1Reliable resultsImprove robustnessBiometric pattern recognitionCounting objects with random distributionCrowd countingCrowds
Owner:RGT UNIV OF MINNESOTA
Remote-controlled programming of a program-controlled device
ActiveUS20060220900A1Jeopardizing confidentialityGuaranteed uptimeComputer controlSimulator controlRemote controlEmbedded system
A method for remote programming of a program-controlled device, and a system having an interface to receive program data and a legitimization, as well as a remotely programmable, program-controlled device, which includes a processor and a program memory, are provided. In the method, program data are remotely transmitted from a control station to the interface and buffer-stored there in a buffer store. Subsequently, a legitimization is transmitted from the control station to the interface, and from there to the program-controlled device. The device checks the legitimization and imports the program data from the buffer store if the legitimization check is positive.
Owner:ROBERT BOSCH GMBH
System and method for capturing, storing, analyzing and displaying data relating to the movements of objects
ActiveUS20100013931A1Easy to compareSmall amount of memoryImage analysisCharacter and pattern recognitionGraphicsTime segment
A system and method for the capture and storage of data relating to the movements of objects, in a specified area and enables this data to be displayed in a graphically meaningful and useful manner. Video data is collected and video metadata is generated relating to objects (persons) appearing in the video data and their movements over time. The movements of the objects are then analyzed to detect the movements within a region of interest. This detection of movement allows a user, such as a manager of a store, to make informed decisions as to the infrastructure and operation of the store. One detection method relates to the number of people that are present in a region of interest for a specified time period. A second detection method relates to the number of people that remain or dwell in a particular area for a particular time period. A third detection method determines the flow of people and the direction they take within a region of interest. A fourth detection method relates to the number of people that enter a certain area by crossing a virtual line, a tripwire.
Owner:COGNYTE TECH ISRAEL LTD
Transcription data extraction
ActiveUS7613610B1Easy to trackEasy searchData processing applicationsAutomatic call-answering/message-recording/conversation-recordingData ingestionMedical record
A computer program product, for performing data determination from medical record transcriptions, resides on a computer-readable medium and includes computer-readable instructions for causing a computer to obtain a medical transcription of a dictation, the dictation being from medical personnel and concerning a patient, analyze the transcription for an indicating phrase associated with a type of data desired to be determined from the transcription, the type of desired data being relevant to medical records, determine whether data indicated by text disposed proximately to the indicating phrase is of the desired type, and store an indication of the data if the data is of the desired type.
Owner:DELIVERHEALTH SOLUTIONS LLC +1
Multi-channel digital wireless audio system
ActiveUS20060153155A1Improve service qualityReduce dataError preventionStereophonic circuit arrangementsComputer hardwareDigital radio
In a multi-channel digital wireless audio system with at least one transmit node and at least one receive node, each node can both receive and transmit digital audio signals. Signals sent from a receive node to a transmit node may acknowledge satisfactory signal receipt, or may requesting retransmission of data packets received in a corrupted state. Original and retransmitted signals may be sent in compressed form to enable use of narrow-band digital radios. The system preferably incorporates a dual control channel to enable transmission of meta data. Each system node preferably incorporates a hardware-multithreaded processor adapted to implement various functions such as baseband functions, RF protocol functions, error correction functions, and audio processing functions, with each independent thread being adapted to implement a different functional block.
Owner:ELEVEN ENG
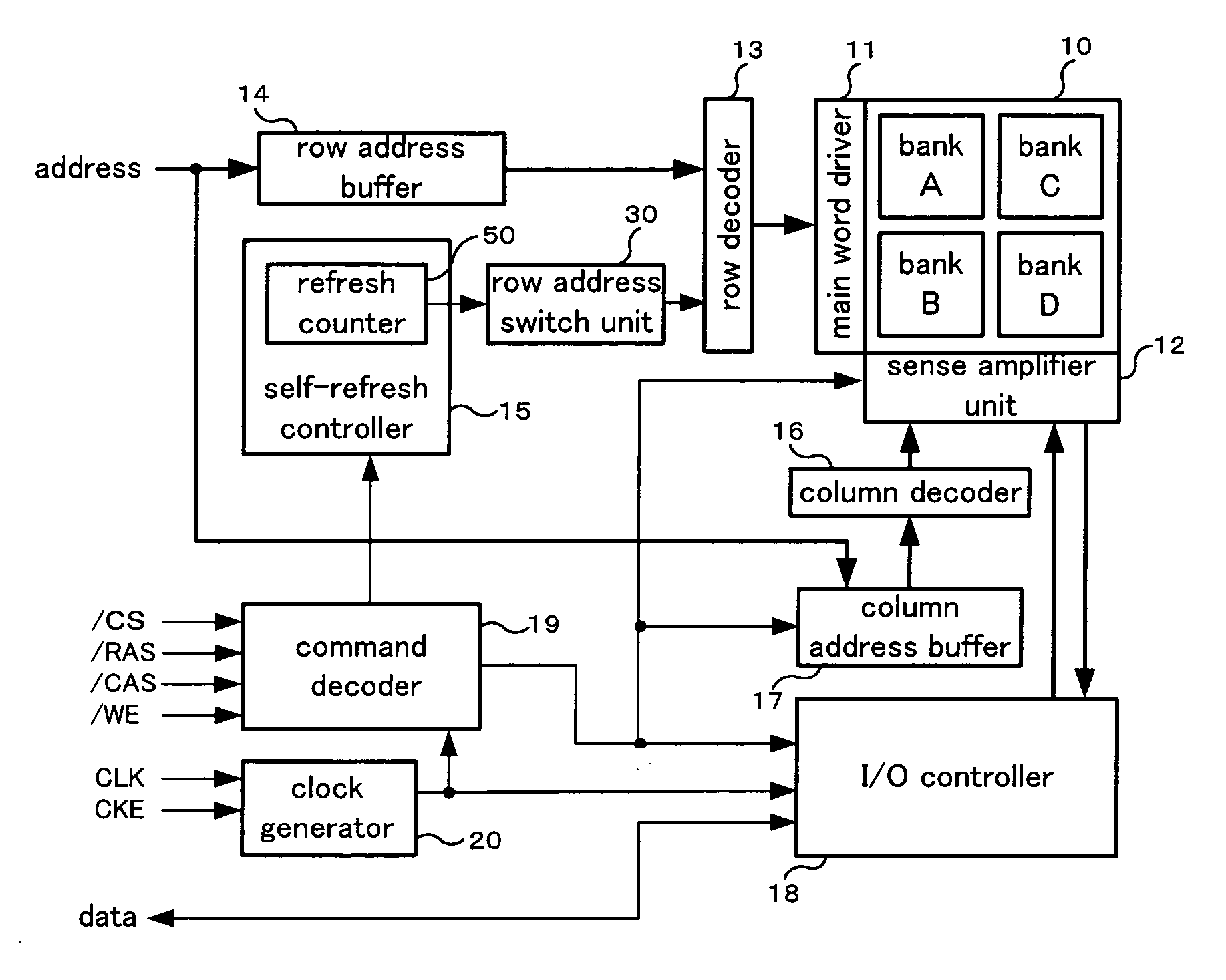
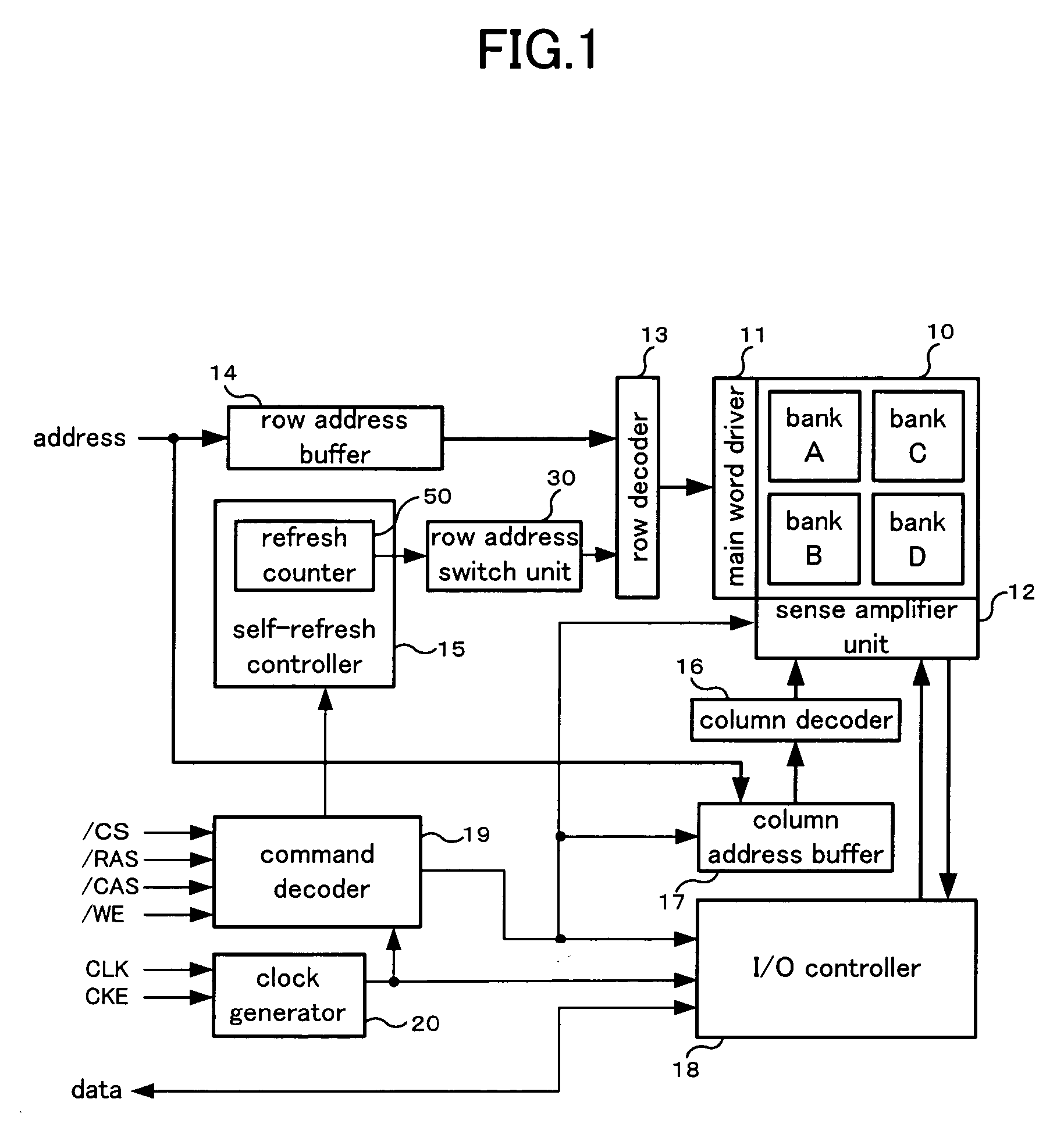
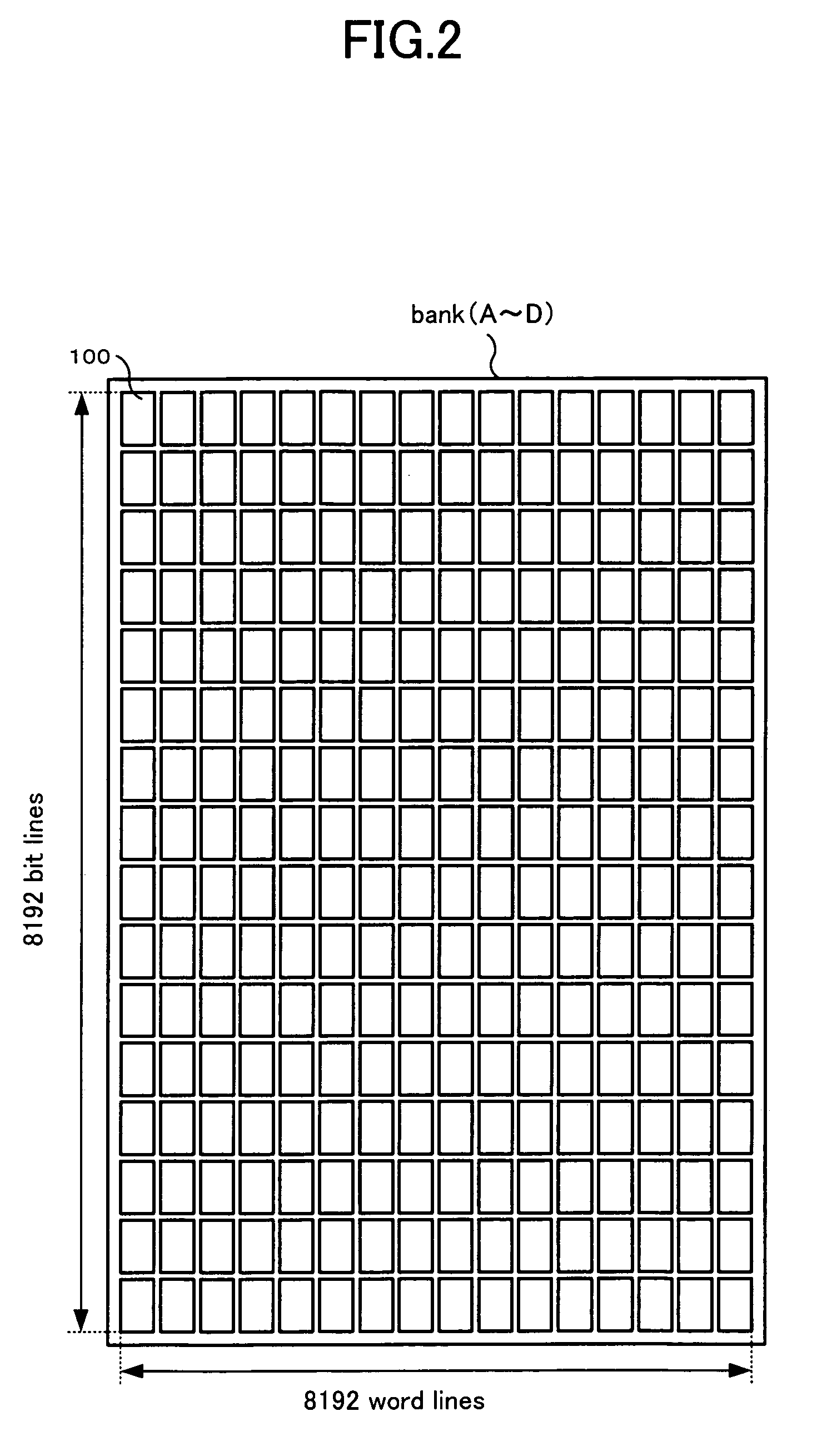
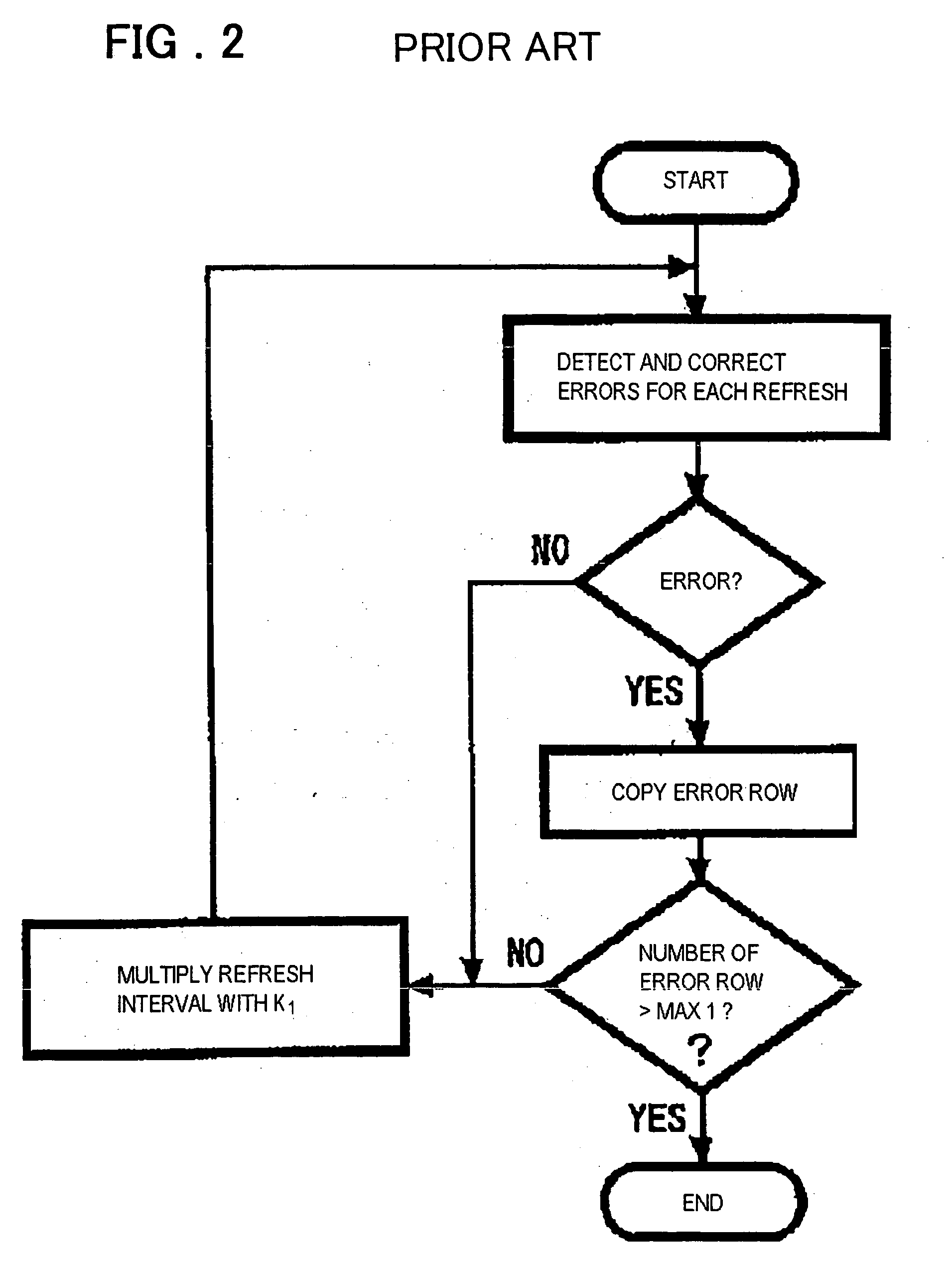
Refresh control method of a semiconductor memory device and semiconductor memory device
A refresh control method of a semiconductor memory device which controls a self-refresh operation to hold data in a memory array having a plurality of memory cells disposed at intersections of word lines corresponding to row addresses and bit lines corresponding to column addresses, comprising: a step for dividing the memory array into a holding area used as a copy source which includes memory cells on a predetermined number of word lines, and a copy area used as a copy destination which includes memory cells on word lines to which entire data of the holding area is to be copied, a step for executing copy operation in which data of each memory cell of the holding area is copied to one or more memory cells in the copy area on the same bit line or the same pair of bit lines before executing the self-refresh operation, and a step for executing the self-refresh operation in which a row address of the holding area is designated and a corresponding word line is selected and driven, and at the same time, one or more word lines in the copy area corresponding to the selected word line are selected and driven.
Owner:PS4 LUXCO SARL
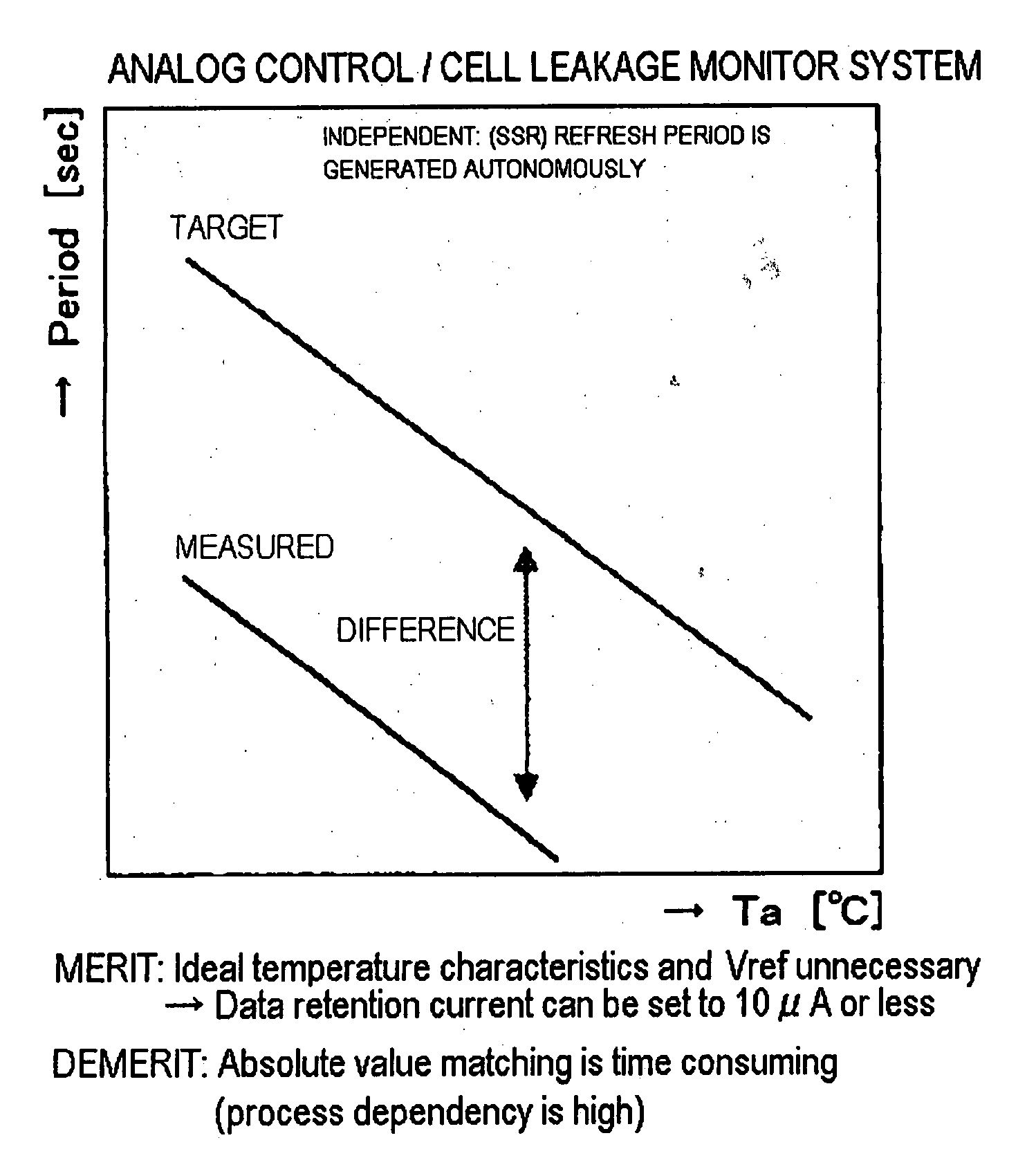
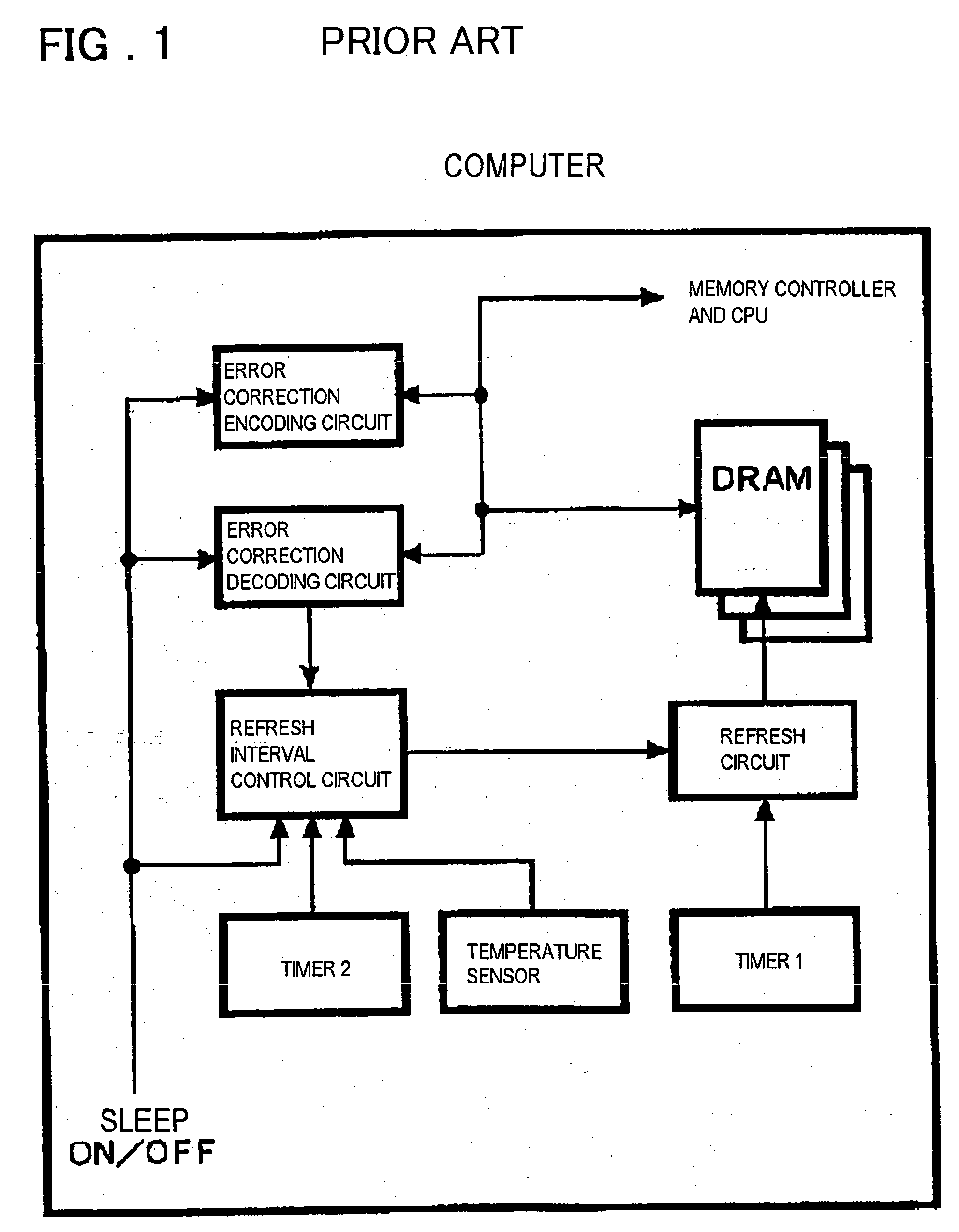
Semiconductor memory device and refresh period controlling method
InactiveUS20050281112A1Improved temperature compensationCost reductionError detection/correctionCode conversionError ratioRate measurement
Disclosed is a memory device including an error rate measurement circuit and a control circuit. The error rate measurement circuit, carrying a BIST circuit, reads out and writes data for an area for monitor bits every refresh period to detect an error rate (error count) with the refresh period. The control circuit performs control for elongating and shortening the refresh period so that a desired error rate will be achieved. The BIST circuit issues an internal command and an internal address and drives the DRAM from inside. The BIST circuit writes and reads out desired data, compares the monitor bits to expected values (error decision) and counts the errors.
Owner:PS4 LUXCO SARL
Progress notes model in a clinical information system
InactiveUS6289316B1Easy to useReduce dataTherapiesPatient personal data managementClinical informationViewpoints
A progress note in a patient's medical record can be created in a database format which is unalterable. New versions of the notes which reflect revised viewpoints of the user may be created without deleting original notes. The database which stores these progress notes can check accuracy in diagnosis and orders by the user.
Owner:UNILOC 2017 LLC
Situation sensitive memory performance
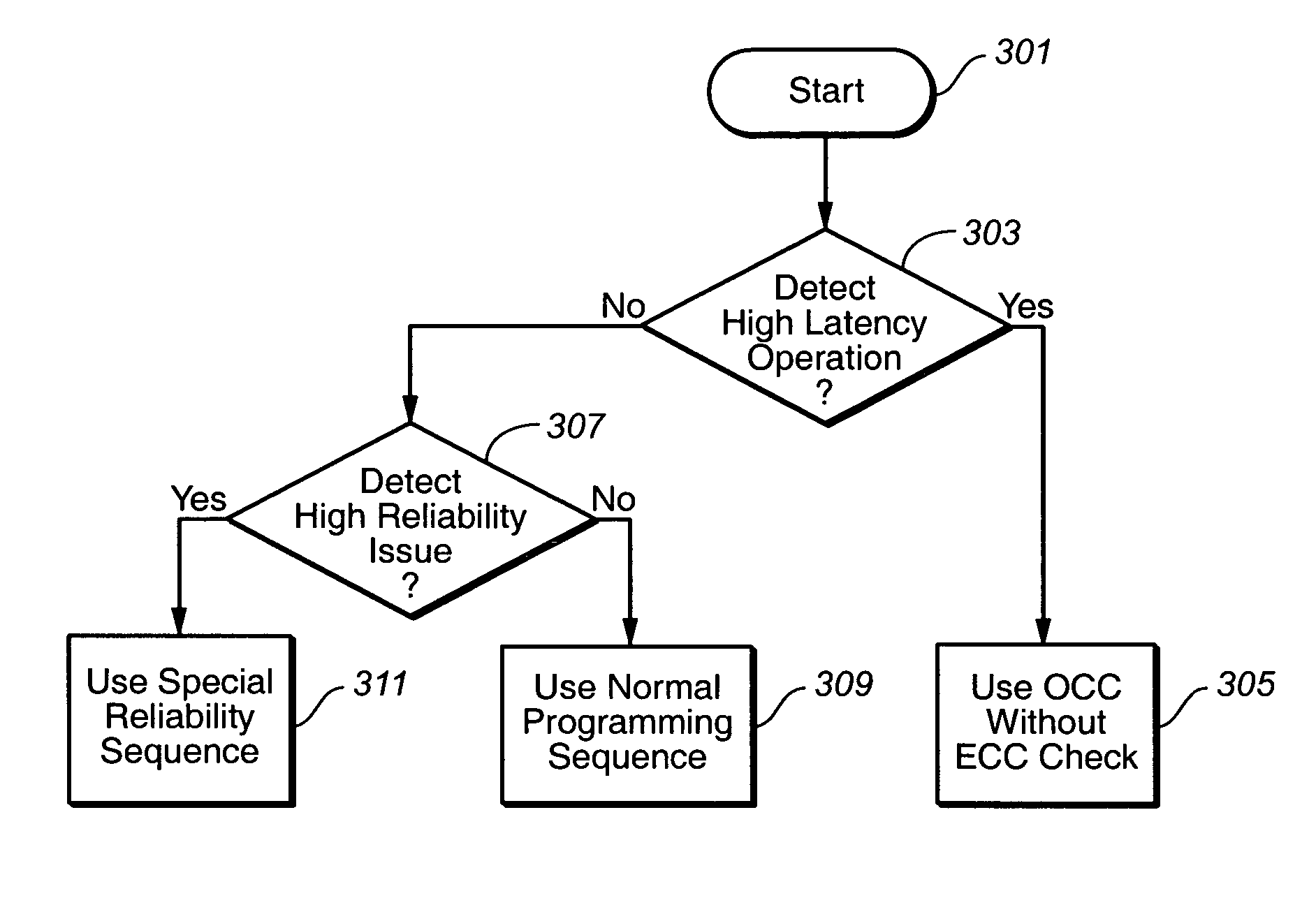
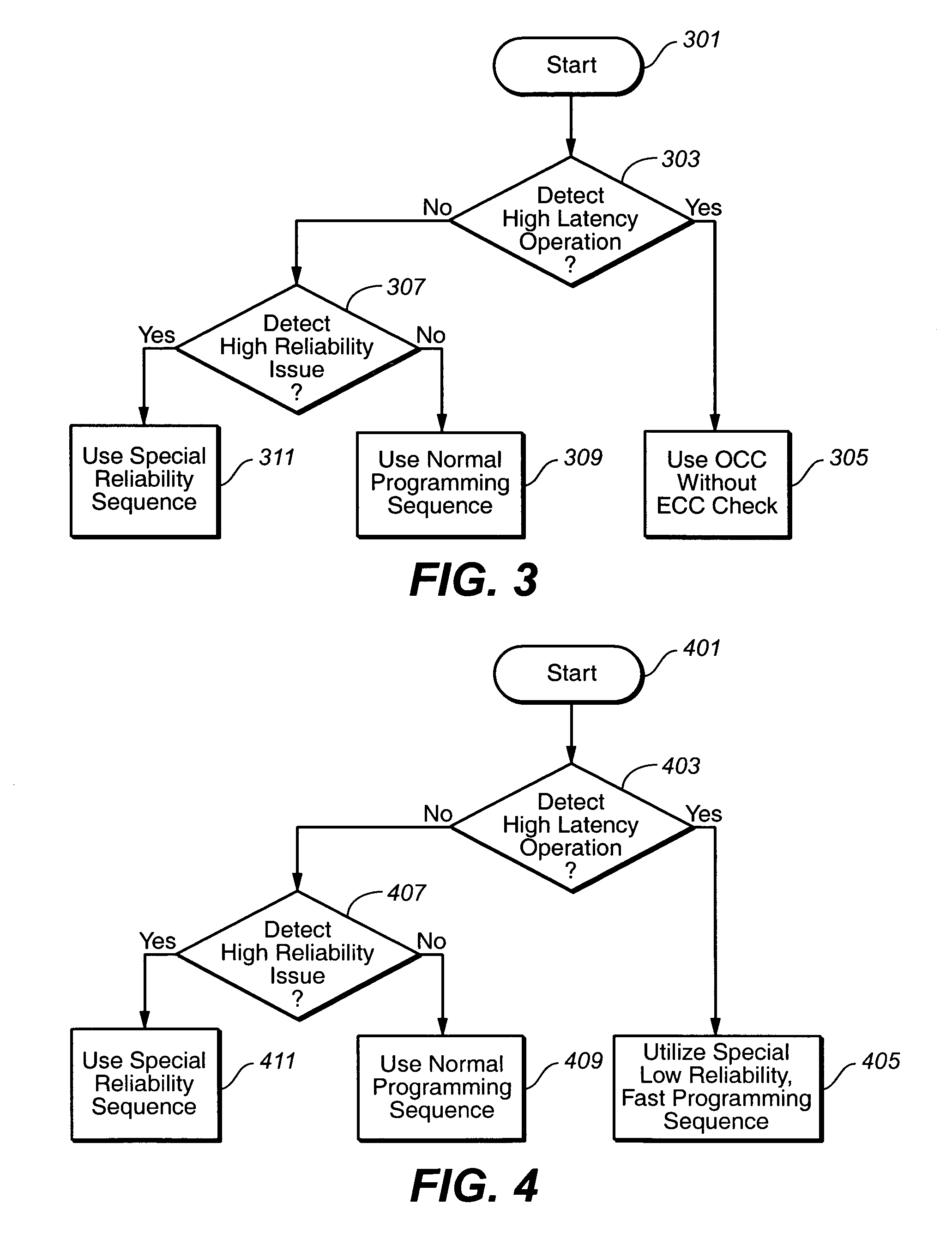
ActiveUS20070033581A1Avoid timeoutImprove programming speedEnergy efficient ICTVolume/mass flow measurementTerm memoryComputer science
The present invention presents a non-volatile memory system that adapts its performance to one or more system related situation. If a situation occurs where the memory will require more than the allotted time for completing an operation, the memory can switch from its normal operating mode to a high performance mode in order to complete the operation quickly enough. Conversely, if a situation arises where reliability could be an issue (such as partial page programming), the controller could switch to a high reliability mode. In either case, once the trigging system situation has returned to normal, the memory reverts to the normal operation. The detection of such situations can be used both for programming and data relocation operations. An exemplary embodiment is based on firmware programmable performance.
Owner:SANDISK TECH LLC
Text-based messaging application cloud
InactiveUS20120240062A1Easy to browseMaximization of overall densityInput/output for user-computer interactionMultiple digital computer combinationsExtensibilityService cloud
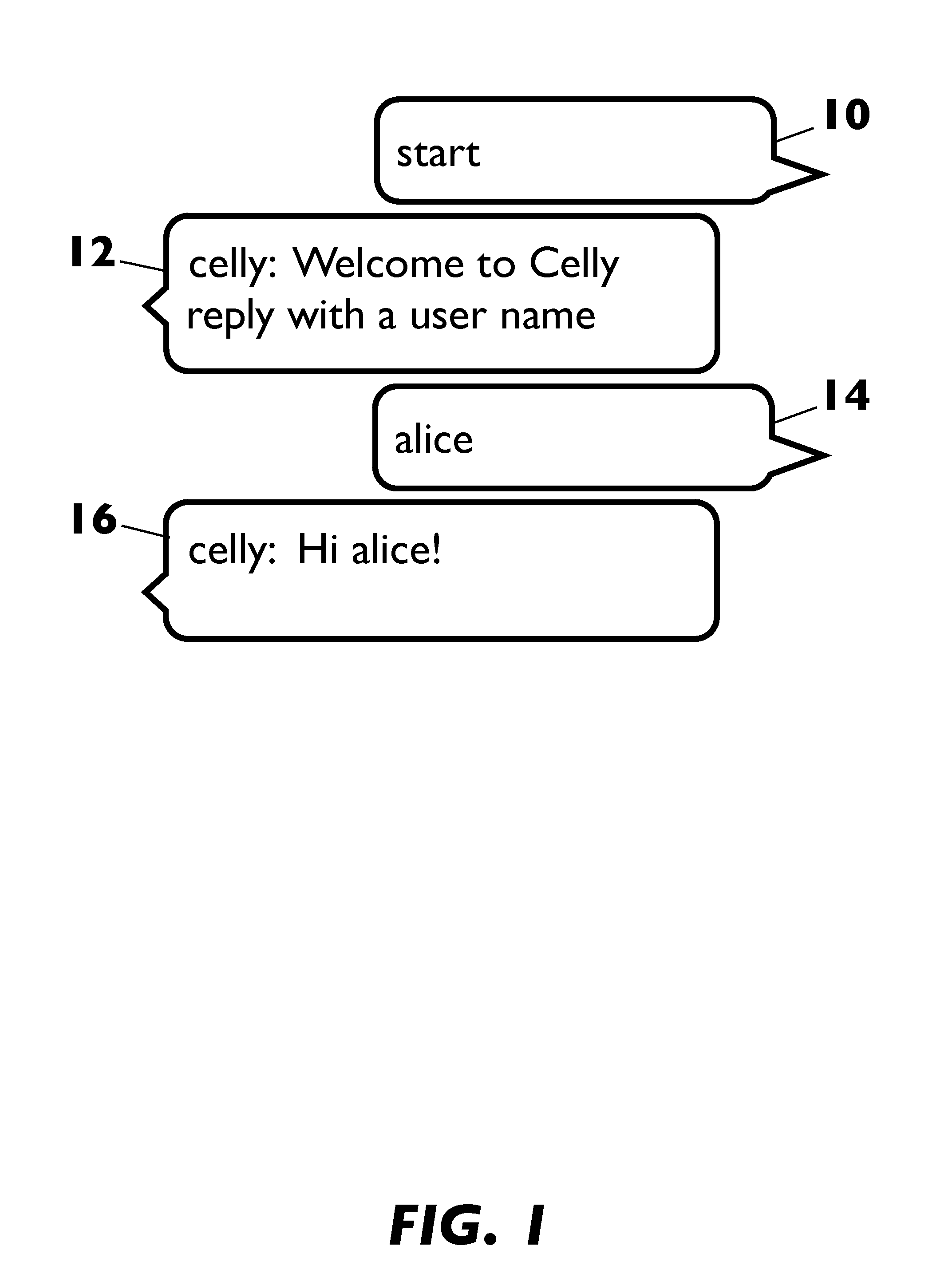
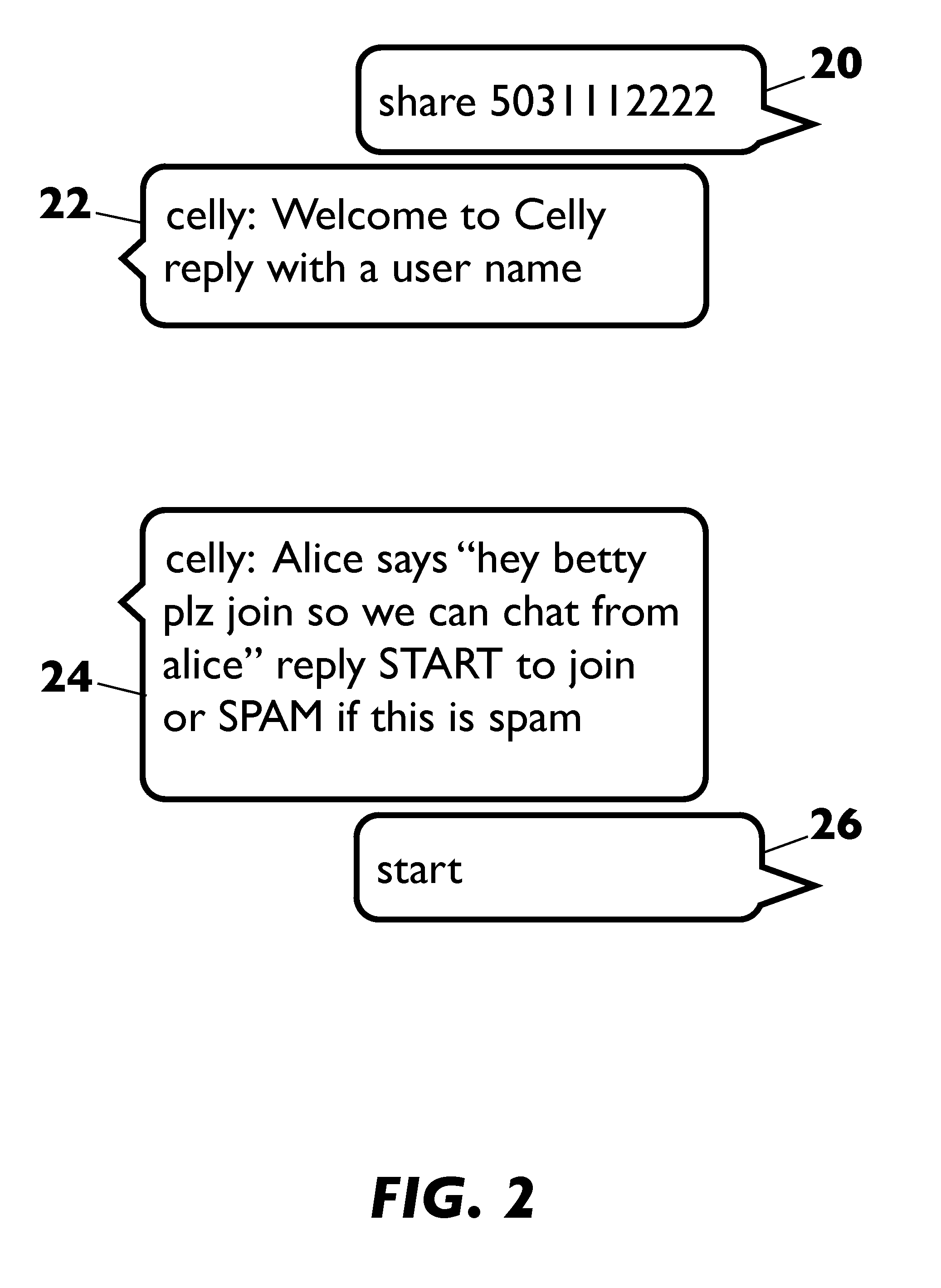
This disclosure relates to systems and methods for providing an application services cloud based on text-based messaging that hosts a plurality of application services that enables applications to be designed and delivered in a simple, productive, and extensible user experience. The present disclosure transforms any device equipped with text-based messaging into a cloud computer, a thin client with connected access to cloud-hosted applications. The disclosure overcomes heretofore unsolved platform design and implementation challenges for text-based messaging applications such as issues of user interface constraints, security, service discovery, information filtering and tracking, platform integration and extensibility, and architectural scalability.
Owner:CELLY
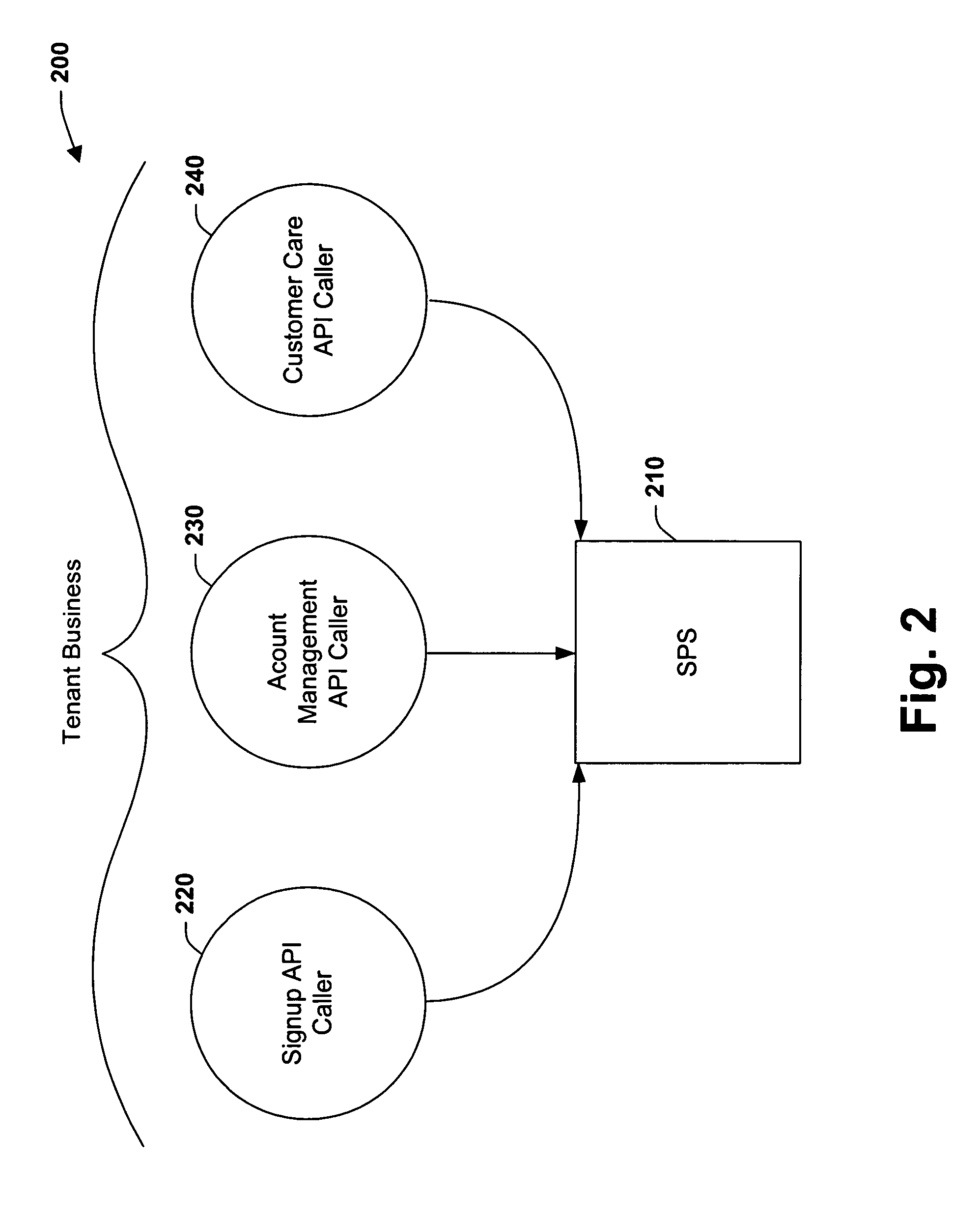
Partner sandboxing in a shared multi-tenant billing system
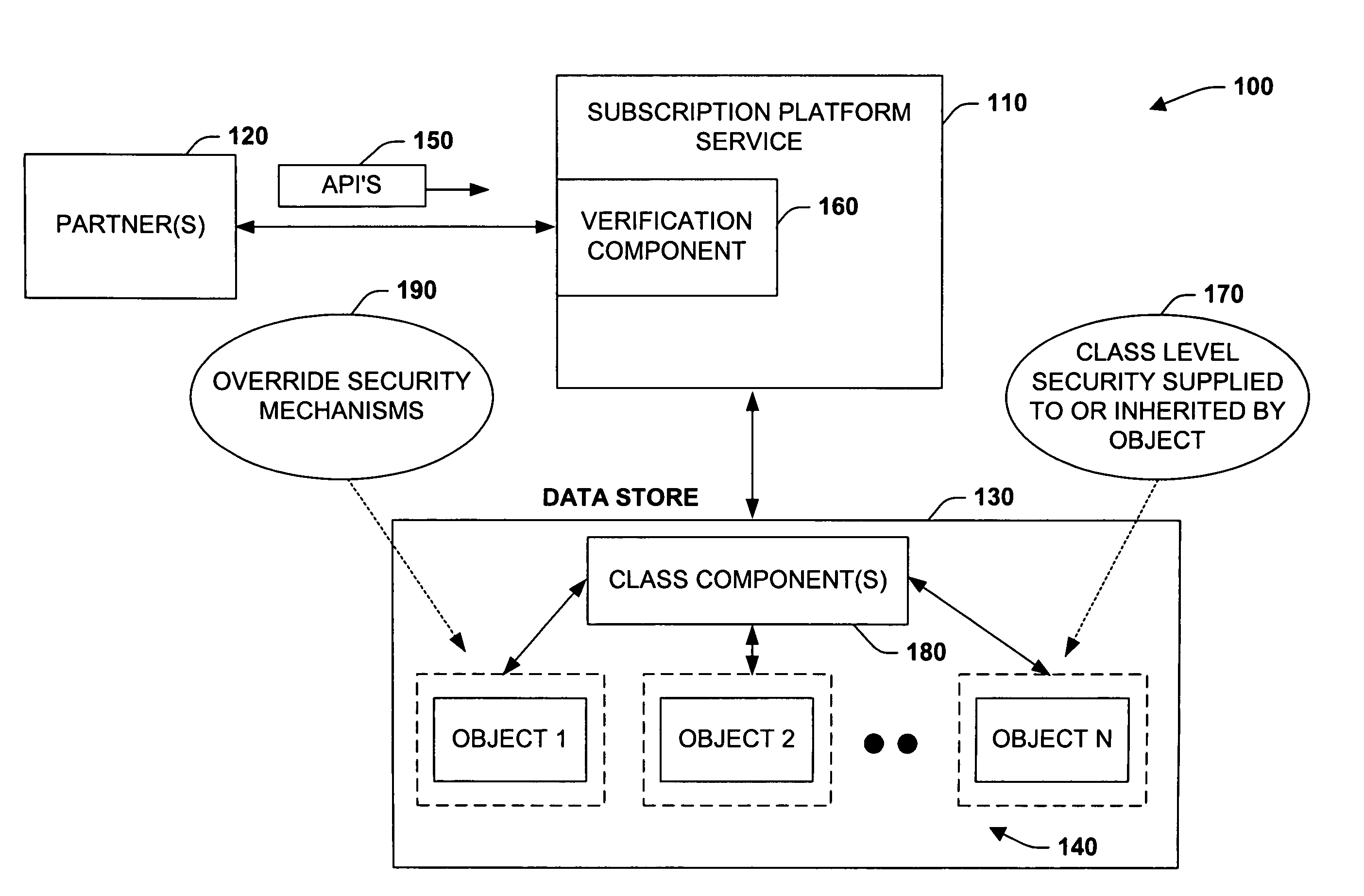
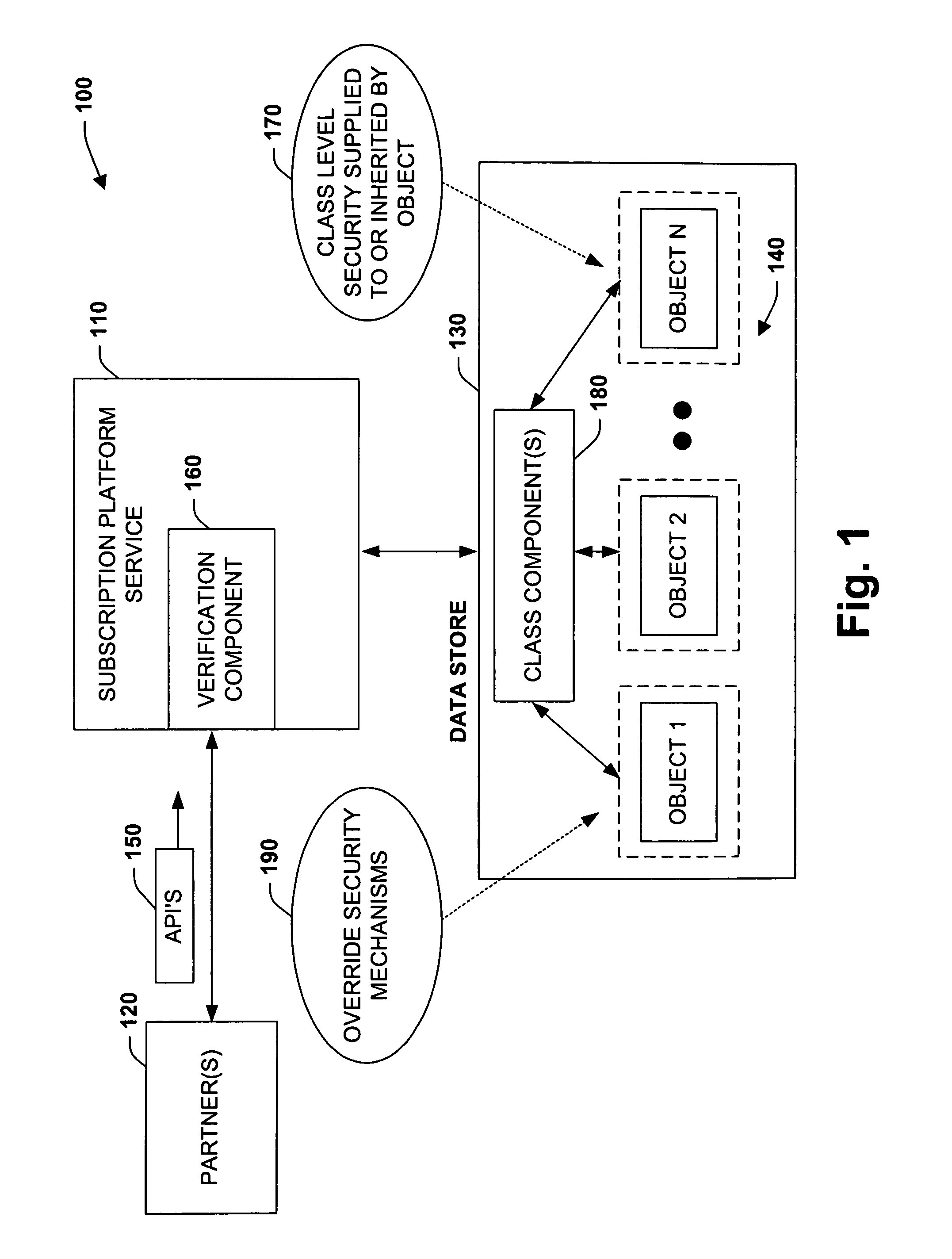
InactiveUS7921299B1Mitigating of confidential dataReduce exposureDigital data processing detailsUnauthorized memory use protectionApplication programming interfaceApplication software
The present invention relates to a system and methodology for interacting with a Subscription Platform Service (SPS) and providing data security between entities that employ such service. The system includes a component that receives a request to access an object by an entity, and a data store that stores security information on classes of the objects. A verification component employs the security information to determine whether the entity has permission to call an Application Programming Interface (API) for the object and / or operate on the object, wherein the verification component exposes the object if permission exists or masks the object if permission does not exist.
Owner:MICROSOFT TECH LICENSING LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com