Novel reagent and kit for renal injury monitoring
A mixture, antibody technology, applied in the field of biotechnology and immunity, can solve the problems of antibody titer difference, unsatisfactory immunogenicity, instability and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the mixture of specific NGAL amino acid fragment sequence and its products as immunogen
[0046] The full-length amino acid sequence of NGAL protein is as follows:
[0047] The full-length amino acid of NGAL protein (human source, 198aa), the sequence is as follows (SEQ ID NO: 3):
[0048] MPLGLLWLGL ALLGALHAQA QDSTSDLIPA PPLSKVPLQQ NFQDNQFQGK
[0049] WYVVGLAGNA ILREDKDPQK MYATIYELKE DKSYNVTSVL FRKKKCDYWI
[0050] RTFVPGCQPG EFTLGNIKSY PGLTSYLVRV VSTNYNQHAM VFFKKVSQNR
[0051] EYFKITLYGR TKELTSELKE NFIRFSKSLG LPENHIVFPV PIDQCIDG
[0052] Due to the disadvantages of difficult synthesis and low immunogenicity of the full-length sequence as an immunogen, the inventors used conventional methods to artificially synthesize sequence fragments of various lengths based on the above full-length sequence, and used the fragments as immunogens to repeatedly test various The immune effect of the immunogen. Finally, a suitable immunogen was obtained, which included...
Embodiment 2
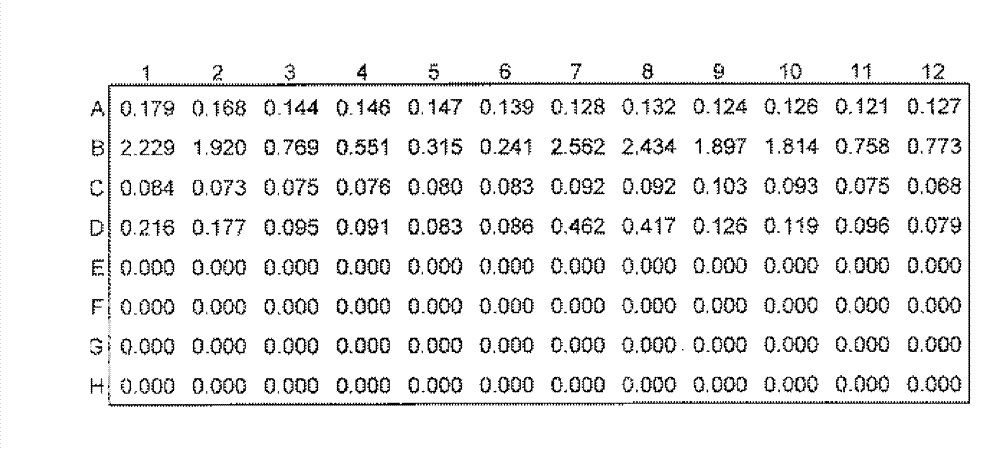
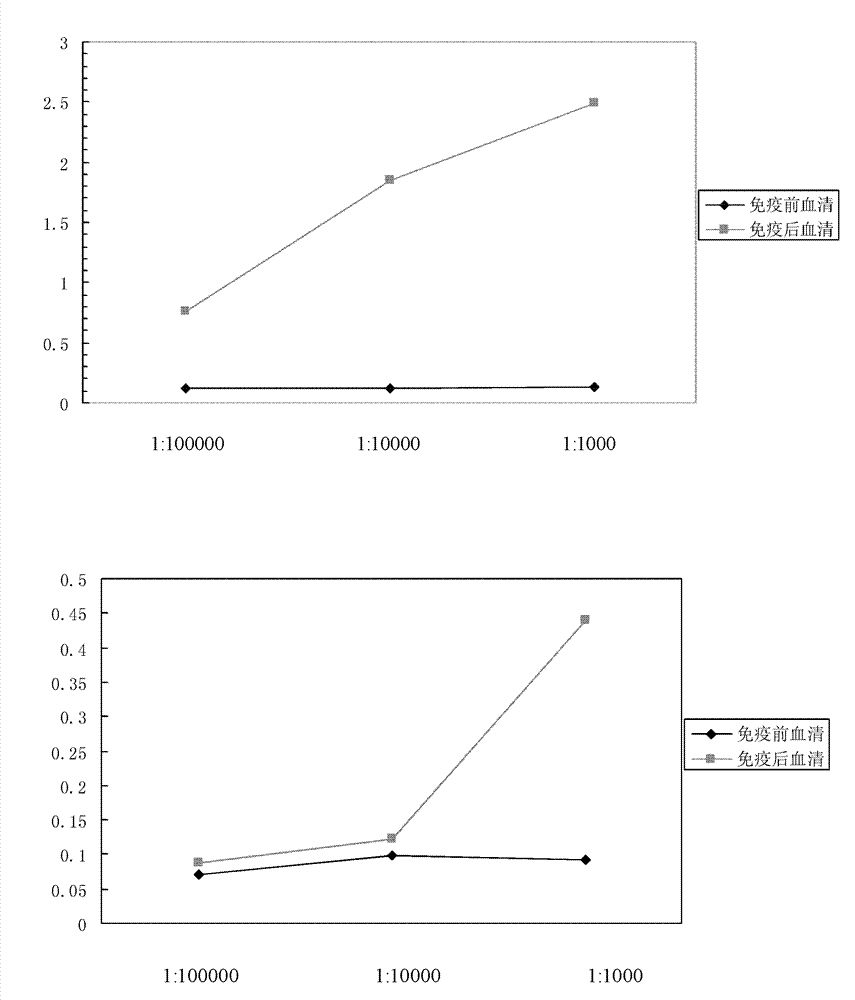
[0056] Example 2, Production of specific polyclonal anti-NGAL antibodies
[0057] The immune sera produced by immunizing New Zealand white rabbits were prepared by using the mixture of specific polypeptides prepared in Example 1 above as immunogens coupled with hemocyanin (KLH). The methods and procedures of animal immunization are as follows:
[0058] 1) Two New Zealand male rabbits (#YZ2391; #YZ2392) were immunized, and the pre-immune serum was collected and stored at -20°C.
[0059] 2) For the first immunization, take the immunogen coupled with hemocyanin (KLH) and the equivalent amount of Freund's complete adjuvant emulsified antigen (antigen: Freund's complete adjuvant = 1:1 (v / v)), multiple points on the back injection. The injection volume for the first immunization was 400 μg of the polypeptide mixture.
[0060] 3) After 14 days, the antigen was emulsified with Freund's complete adjuvant (antigen: Freund's complete adjuvant = 1:1 (v / v)) and repeated back multi-point...
Embodiment 3
[0085] Embodiment 3, test kit
[0086] Described test kit comprises:
[0087] Container 1, and the polyclonal antibody prepared in the aforementioned Example 2 contained in the container;
[0088] Container 2, and the horseradish peroxidase-labeled goat anti-human IgG polyclonal antibody contained in the container.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com