Preparation method and method of hog cholera-ring-mixed antigen, hog cholera-ring subunit vaccines and preparation method thereof
A technology of mixing antigens and doublets, which is used in biochemical equipment and methods, medical preparations containing active ingredients, vaccines, etc., can solve the problems of insufficient antigen concentration, cumbersome preparation process, affecting immune effect, etc., and saves money. The effect of human and material resources, high antibody titer and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
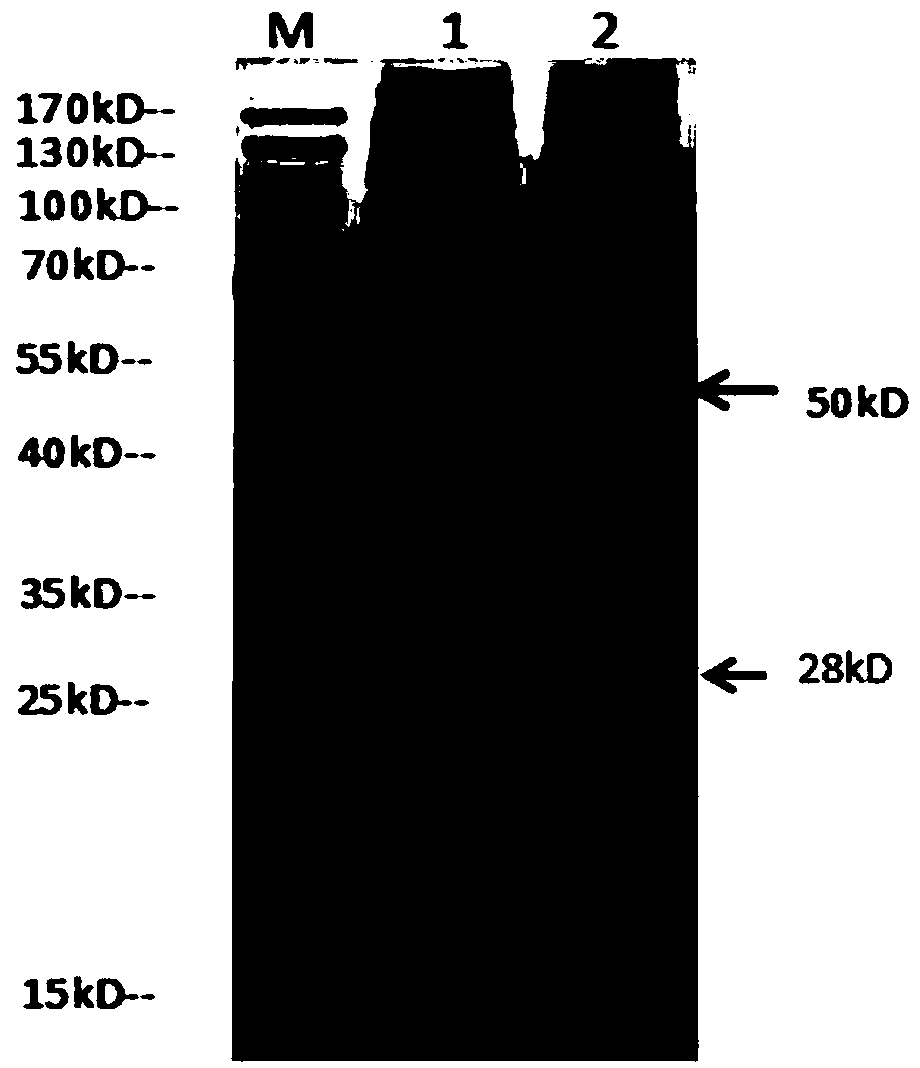
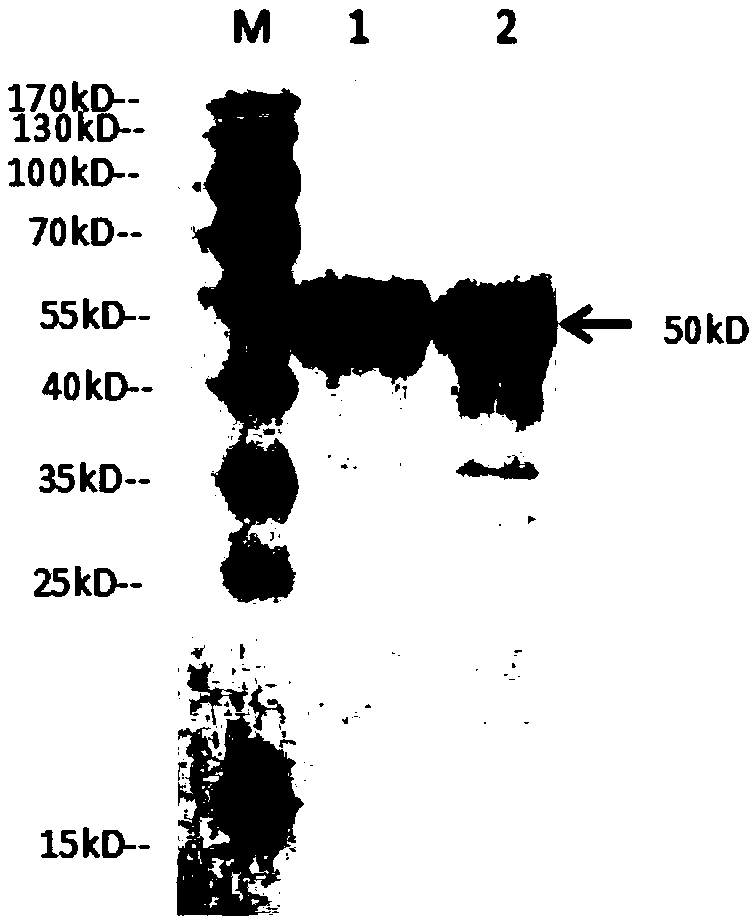
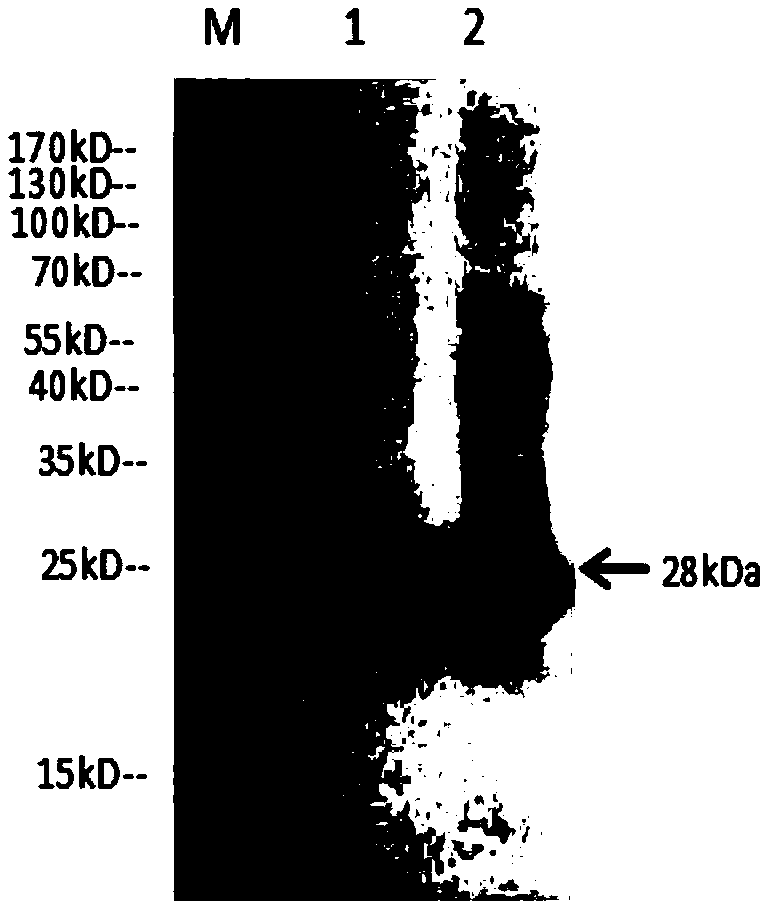
Image
Examples
preparation example Construction
[0048] According to a first aspect of the present invention, a method for preparing a hog fever-circle mixed antigen is provided, comprising the steps of:
[0049] Inoculate the virus mixture of the classical swine fever E2 protein recombinant virus and the porcine circovirus type 2 Cap protein recombinant virus in the cell culture to obtain the mixed antigen.
[0050] Classical swine fever is an acute, febrile, contagious infectious disease caused by the swine fever virus of the family Flaviviridae, which is highly contagious and lethal, and can occur all year round. Swine fever is currently one of the key diseases in livestock diseases, and the outbreak of the disease is likely to cause heavy losses in the breeding industry.
[0051] Porcine circovirus disease, caused by porcine circovirus, has a variety of clinical symptoms, such as anorexia, dyspnea or sluggishness, and has a high mortality rate. It is also one of the key diseases in livestock diseases. one.
[0052] The...
Embodiment 1
[0121] Co-cultivation of recombinant baculovirus with classical swine fever E2 protein and recombinant baculovirus with porcine circovirus type 2 Cap protein: Add shake flasks to 14L tanks to culture 2.5L with a density of about 1 million / ml and cell viability greater than 95 % more than Sf-9 cells, adjust the cell density in the reaction tank to about 1 million / ml by adding fluid. The dissolved oxygen (Do) in the bioreactor was set at 40%, the temperature was 27° C., the pH was 6, the volume of the cell culture in the reactor was controlled to be 10 L, and the rotation speed was 100 rpm. When the cell density of the 14L tank is about 1 million / ml, insert classical swine fever E2 baculovirus and PCV2 recombinant baculovirus according to the amount of MOI=0.1. The operating parameters of the reactor were recorded every day, and samples were taken and counted on the 3rd to 4th day. Take a sample every day afterwards, calculate the cell viability rate with a cell counter, collec...
Embodiment 2
[0126] The only difference from Example 1 is that the virus mixture of the recombinant baculovirus of classical swine fever E2 protein and the recombinant baculovirus of porcine circovirus type 2 Cap protein is not obtained by joint culture, but after being cultured separately, the virus is harvested, and then All were inoculated in High5 cell culture at MOI=0.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com