A kind of tumor-specific antigen and its application
A tumor-specific, antigen-based technology for biomedical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
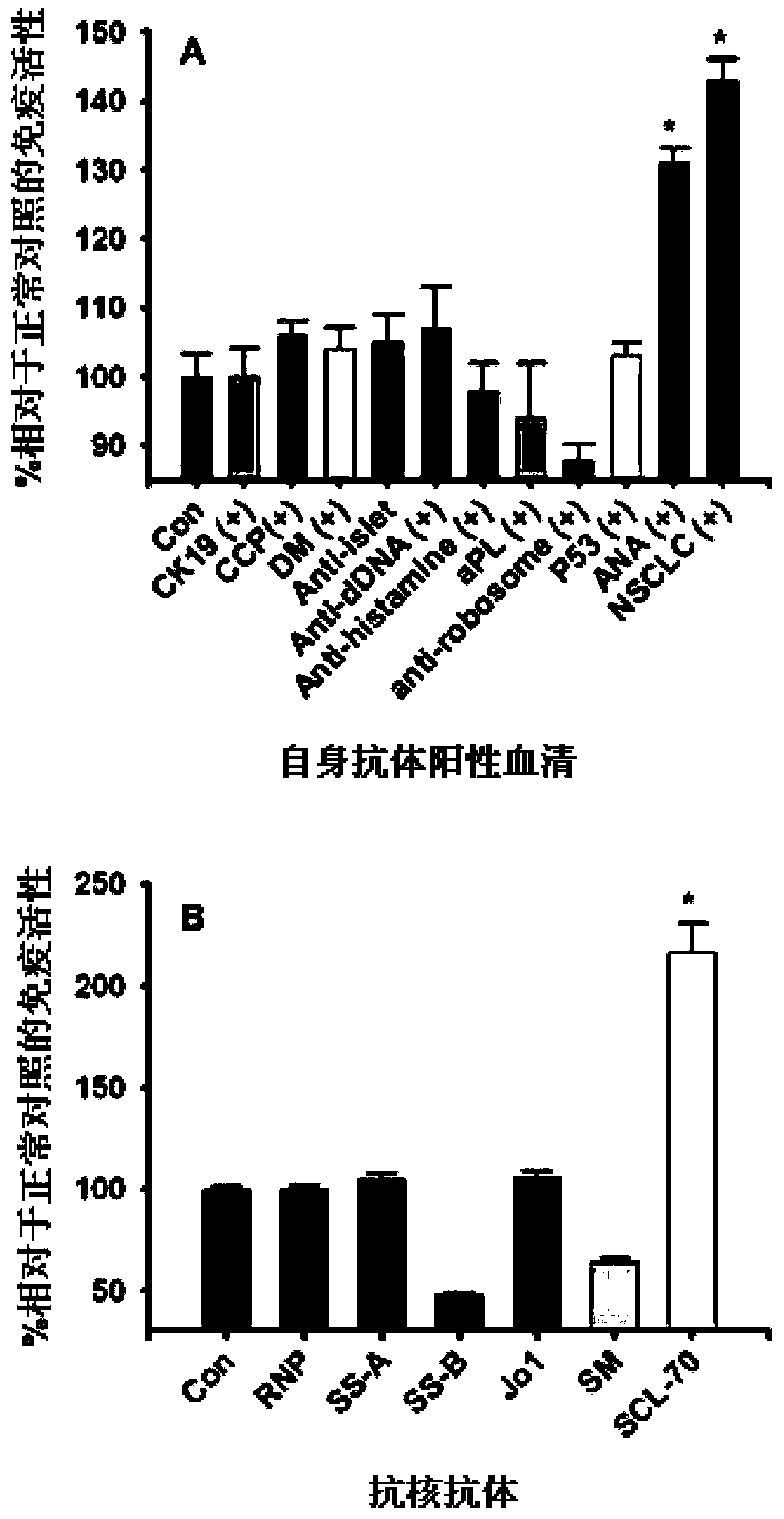
[0039] Example 1 Separation, purification and screening of tumor-specific antigen TOP-1-40
[0040] 1. Materials and Methods
[0041] (1) Tissue homogenate:
[0042] The lung cancer tissue was homogenized in lysate (0.5% NP40, 0.15M NaCl, 5mM EDTA, 50mM Tris, 1mMPMSF) with a tissue homogenizer. Then centrifuge at 12000 rpm at 4°C, and combine the supernatants.
[0043] (2) Separation of tumor antigens by two-dimensional electrophoresis:
[0044] The experiment included isoelectric focusing electrophoresis in the first dimension and SDS-PAGE electrophoresis in the second dimension. The first-dimension isoelectric focusing electrophoresis steps are as follows:
[0045] ·Take the hydration loading buffer (1ml / tube) frozen at -20°C and dissolve at room temperature. Add 0.01g DTT and Bio-Lyte 4-72.5ml to the small tube, and mix well.
[0046] ·Remove 400ml from the mixed hydration loading buffer, add 100ml sample, and mix well.
[0047] · Take the IPG prefabricated gel strip...
Embodiment 2
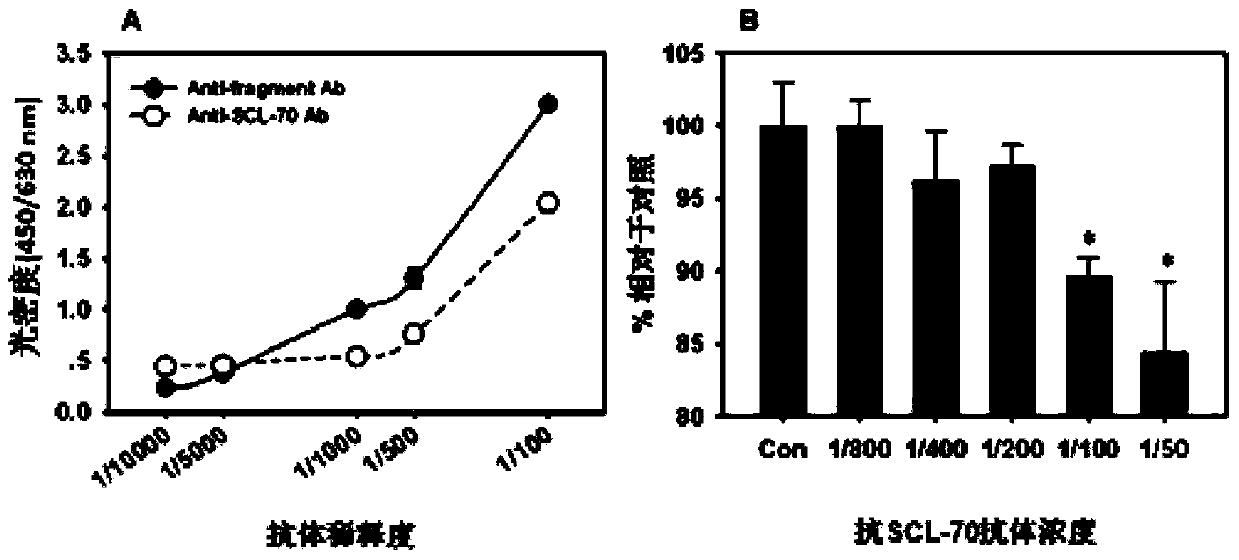
[0099] Example 2 Preparation of rabbit anti-human TOP-1-40 polyclonal antibody
[0100] 1. Materials and Methods
[0101] Use the target protein separated and purified by two-dimensional electrophoresis as an immunogen.
[0102] • Dilute the target protein to 500 μg / ml with physiological saline. For the first time, it was mixed with complete Freund's adjuvant at a ratio of 1:1, and then immunized by subcutaneous injection in New Zealand white rabbits. Afterwards, mix the purified target protein with incomplete Freund's adjuvant at a ratio of 1:1, and continue to immunize animals, with an interval of 1-2 weeks, for 4 consecutive times. The antibody titer against the target protein was detected by antibody capture enzyme-linked immunosorbent method, and after the titer reached a certain height, a booster immunization was carried out at the end.
[0103] · Three days later, blood was collected from the inferior vena cava of the animal, and the serum was separated by centrifuga...
Embodiment 3
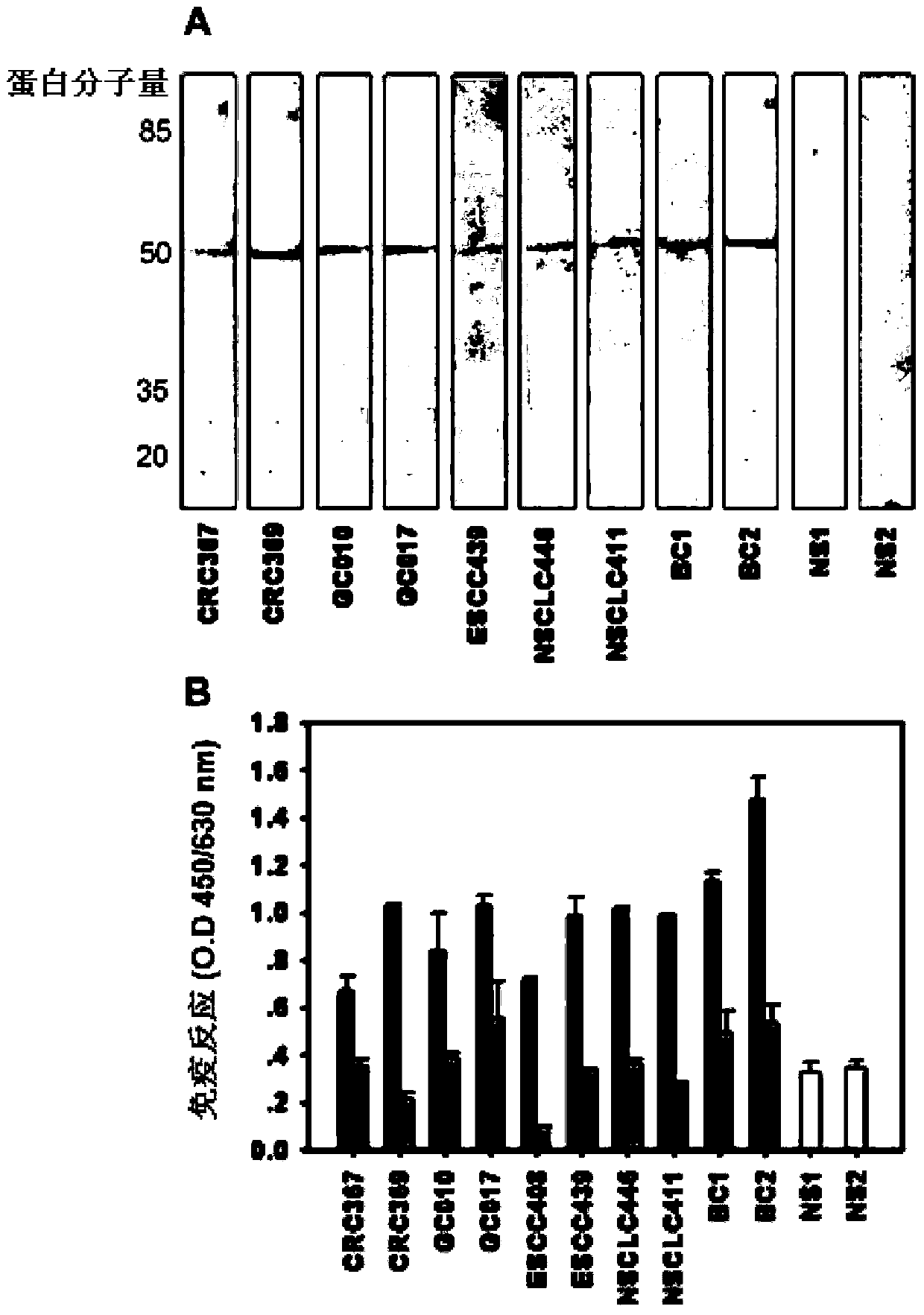
[0109] Example 3 Immunohistochemical detection of the content of TOP-1-40 in cancer
[0110] 1. Materials and Methods
[0111] Cancer tissue samples for this study included colorectal cancer (40 cases), non-small cell lung cancer (20 cases), gastric cancer (20 cases), breast cancer (35 cases), endometrial cancer (20 cases), esophageal cancer ( 15 cases), ovarian cancer (10 cases), liver cancer (10 cases) and bladder cancer (10 cases), and corresponding normal tissues. These samples were all confirmed by pathological examination of Department of Pathology, Sichuan Provincial People's Hospital.
[0112] Using anti-TOP-1-40 polyclonal antibody, the expression of TOP-1-40 in various cancer tissues was detected by conventional immunohistochemical method and compared with corresponding normal tissues. The specificity of the anti-TOP-1-40 polyclonal antibody has been demonstrated by Western blotting and immunofluorescence (see Example 2).
[0113] The operation of the immunohistoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com