Poliomyelitis inactivated vaccine and its production method
A technology for polio and inactivated vaccine, which is applied in the field of inactivated polio vaccine and its production, can solve the problems of low virus titer in harvested liquid and no disclosure of culture conditions, and achieves high cell inoculation density, uniform quality, Highly controllable effect of batch-to-batch variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Experiment of culturing poliovirus in basket reactor under different culture medium and pH value conditions
[0037] 1 Experiment of culturing poliovirus under different media and different pH values of each media
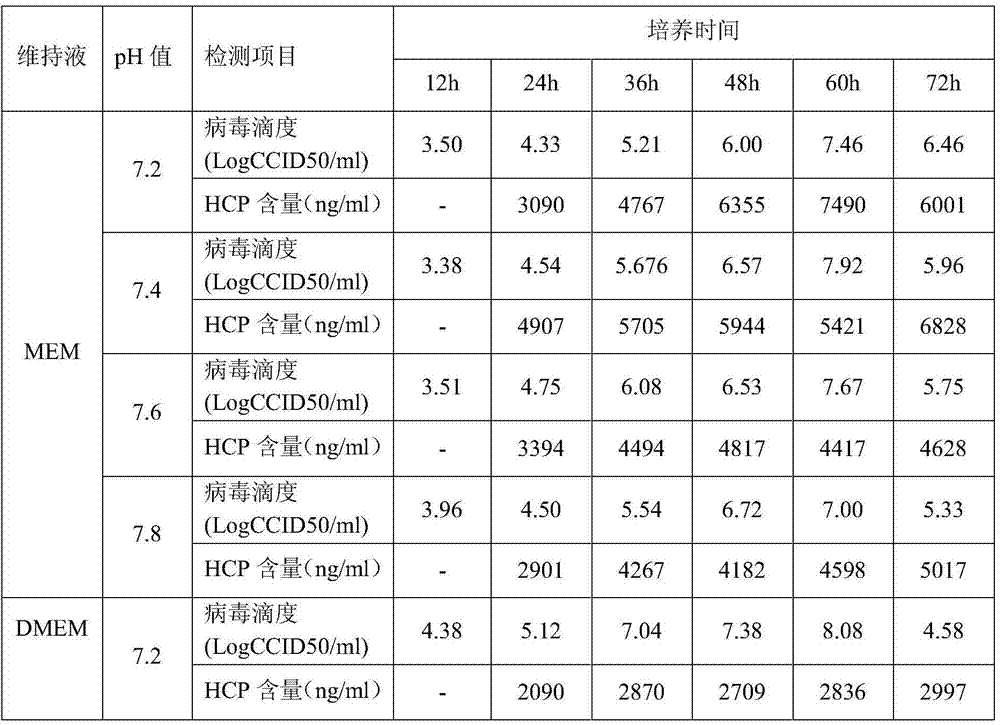
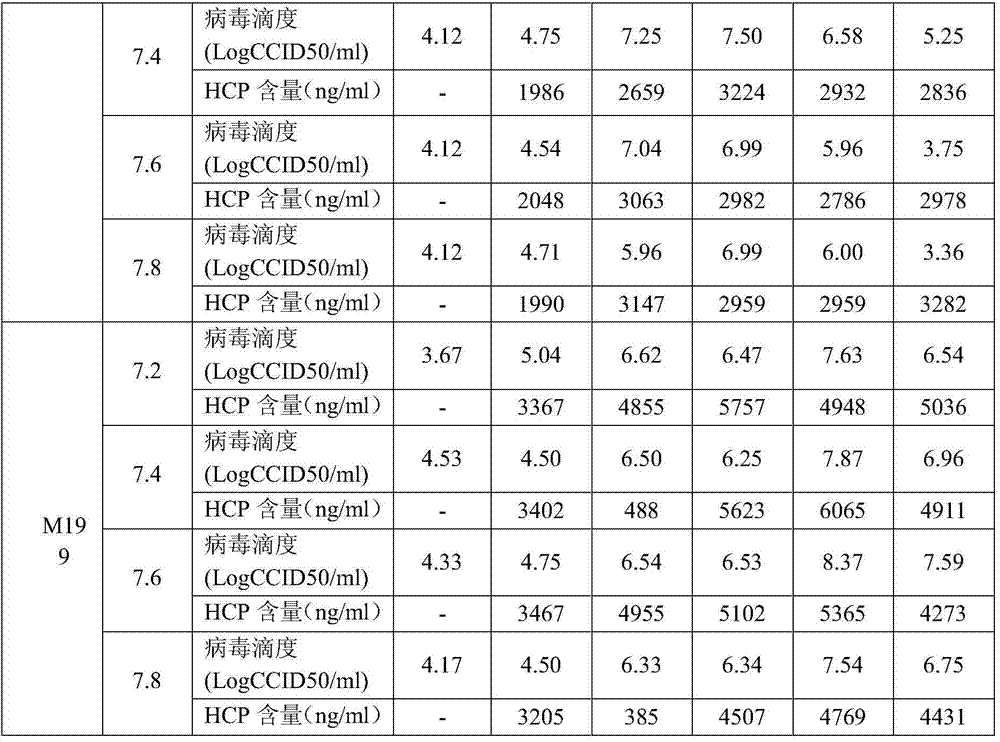
[0038] M199, DEME and MEM were used to prepare cell growth solution and virus maintenance solution. Each medium was prepared into 4 pH gradients, pH 7.2, pH 7.4, pH 7.6 and pH 7.8, respectively. Vero cells were inoculated for culturing. After the cells were cultured for 6 to 8 days and grew into a dense monolayer, they were washed three times with Earle's solution, and were inoculated with I, II, and III polioviruses at the same MOI (MOI=0.01) for culture. , the virus titer and the residual protein (HCP) content of Vero cells were determined at different times of culture.
[0039] 1.1 Culture test of type I poliovirus under different medium and pH conditions
[0040] When type I poliovirus was cultured in M199, DEME and MEM, the viral titers of ...
Embodiment 2
[0063] Embodiment 2. Cultivation test of different culture temperature and MOI of poliovirus in basket reactor
[0064] 1. Preliminary screening of poliovirus culture temperature and MOI conditions in disposable cell flasks
[0065] First, static culture experiments were carried out in disposable cell square flasks. Referring to the culture temperature of oral poliovirus (33±0.5°C), two different temperatures, 33±0.5°C and 31±0.5°C, were selected for poliovirus culture. Three different MOIs, namely 0.01, 0.1 and 0.3, were used to detect the virus titer and HCP content at different temperatures and different MOIs.
[0066] 1.1 Culture test of type I poliovirus at different culture temperature and MOI
[0067] Under the culture condition of 33℃, the virus titer of type I poliovirus was better than that of 31℃, and there was basically no difference in the level of HCP; under the three different MOI culture conditions selected, the virus reproduction peak could be reached for 60...
Embodiment 3
[0093] Example 3. Determination of Dissolved Oxygen for Poliovirus Culture in Basket Reactor
[0094] The basket reactors used three different dissolved oxygen (DO) settings of 30%, 50% and 70%, which corresponded to dissolved oxygen (DO) ranges of 20-40%, 40-60% and 60-80%. Three types of poliovirus were cultured, and the changes of virus titer under different dissolved oxygen saturation were compared to determine the appropriate DO for culturing poliovirus in the basket reactor. For other culture conditions, refer to Example 2.
[0095] The test results showed that when the DO was 30%, the virus titers of the three types were far lower than the virus titers when the DO was 50% and 70%. There was no significant difference in % virus titer, so the optimal dissolved oxygen range was determined to be 40-80%. The results are shown in Table 11 to Table 13.
[0096] Table 11 Virus titers of type I poliovirus under different dissolved oxygen
[0097] time
DO = 30% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com