Newcastle disease and H9N2 subtype avian influenza bivalent inactivated vaccine and preparation method thereof
A dual inactivated vaccine, a technology for chicken Newcastle disease, which is applied in the field of dual inactivated chicken Newcastle disease and H9N2 subtype avian influenza vaccine and its preparation, can solve the problems of low cross-immunization protection rate, large immune dose, low antibody level, and the like, Achieve the effect of no formaldehyde residue, high antibody level and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Isolation and Identification of Example 1 H9N2 Subtype Avian Influenza Virus HN03 Strain
[0025] 1. Disease data
[0026] The disease material is aseptically collected heart blood, liver, brain and lung tissues of dead sick chickens.
[0027] 2. Tissue contacts
[0028] The disease materials were used as heart blood smears and viral tissue contact slides of various organs, and stained microscopic examination was performed, and the staining microscopic examination results of viral tissue contact slides were negative.
[0029] 3. Bacterial Culture
[0030] The above disease materials were cultured with Martin broth and sheep blood culture medium (prepared according to the method in the appendix of the current "Chinese Veterinary Pharmacopoeia"), and the results showed no colony growth, indicating that the disease materials did not contain bacteria.
[0031] 4. Treatment of disease materials
[0032] Cut and grind the above disease material in a sterile container, an...
Embodiment 2
[0058] Example 2 Preparation of Chicken Newcastle Disease and H9N2 Avian Influenza Dual Inactivated Vaccine
[0059] 1. Source of poisonous seeds for seedling production:
[0060] The virus species used to manufacture this product are chicken Newcastle disease virus La Sota strain and avian influenza virus A / chicken / He Nan / 03 / 2009 / (H9N2) strain CGMCC NO: 6258, of which avian influenza virus A / chicken / He Nan / 03 / The 2009 / (H9N2) strain is abbreviated as the H9N2 subtype avian influenza virus HN03 strain.
[0061] Chicken Newcastle Disease Virus La Sota strain (China Veterinary Drug Control Institute strain number: CVCC AV1615 strain) was purchased from China Veterinary Drug Control Institute, H9N2 subtype avian influenza virus HN03 strain was obtained from the National Veterinary Biological Products Engineering Technology Research Center according to Example 1 The method was isolated, identified by the Harbin Veterinary Research Institute, and submitted to the General Microbi...
Embodiment 3
[0099] Example 3 Double-dose Inoculation Safety Test of Chicken Newcastle Disease and H9N2 Avian Influenza Dual Inactivated Vaccine
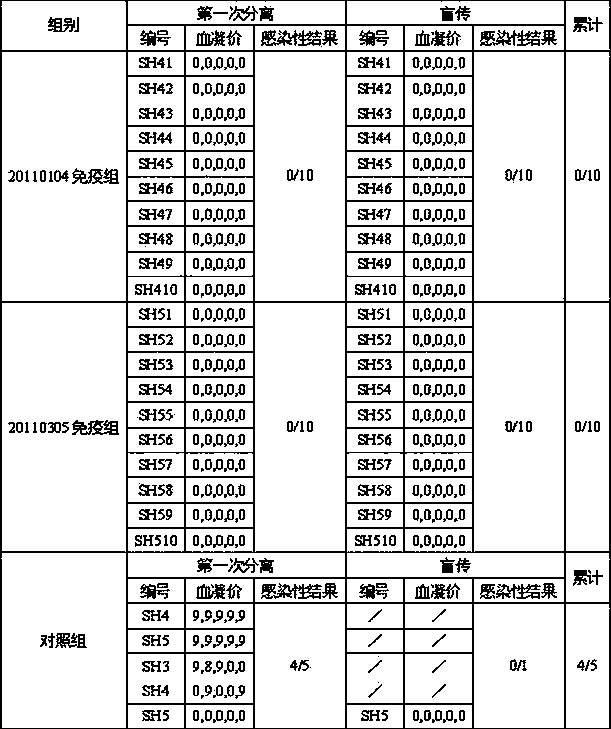
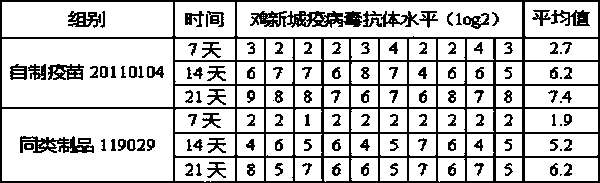
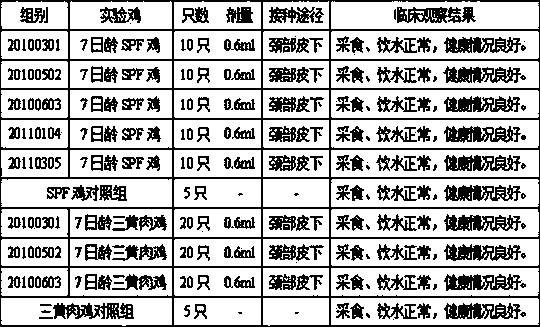
[0100] Prepare 5 batches of chicken Newcastle disease and H9N2 avian influenza dual inactivated vaccine according to Example 2 (batch numbers: 20100301, 20100502, 20100603, 20110104, 20110305 ) , before inactivation, the antigen content of Newcastle disease virus La Sota strain and avian influenza (H9 subtype) HN03 strain were both 10 8.0 EID 50 / 0.3ml / feather portion.
[0101] The research objects were 7-day-old SPF chickens (purchased from Beijing Merial Weitong Experimental Animal Co., Ltd.) and 7-day-old Sanhuang broiler chickens (purchased from Nanjing Shifosi Chicken Farm). After each batch of chickens was bought back, they were adapted for a few days to check the general condition and clinical disease status of the chickens. Weak chickens were eliminated and healthy chickens were selected to enter the test. Five batches of double-dos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com