Swine pasteurellosis vaccine enrichment medium
An enrichment culture medium, a technology for swine pneumonia, which is applied in the field of biology, can solve the problems of low survival rate of bacterin freeze-drying, low number of cultured bacteria, precipitation in the culture medium, etc. The effect of high bacterial count and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
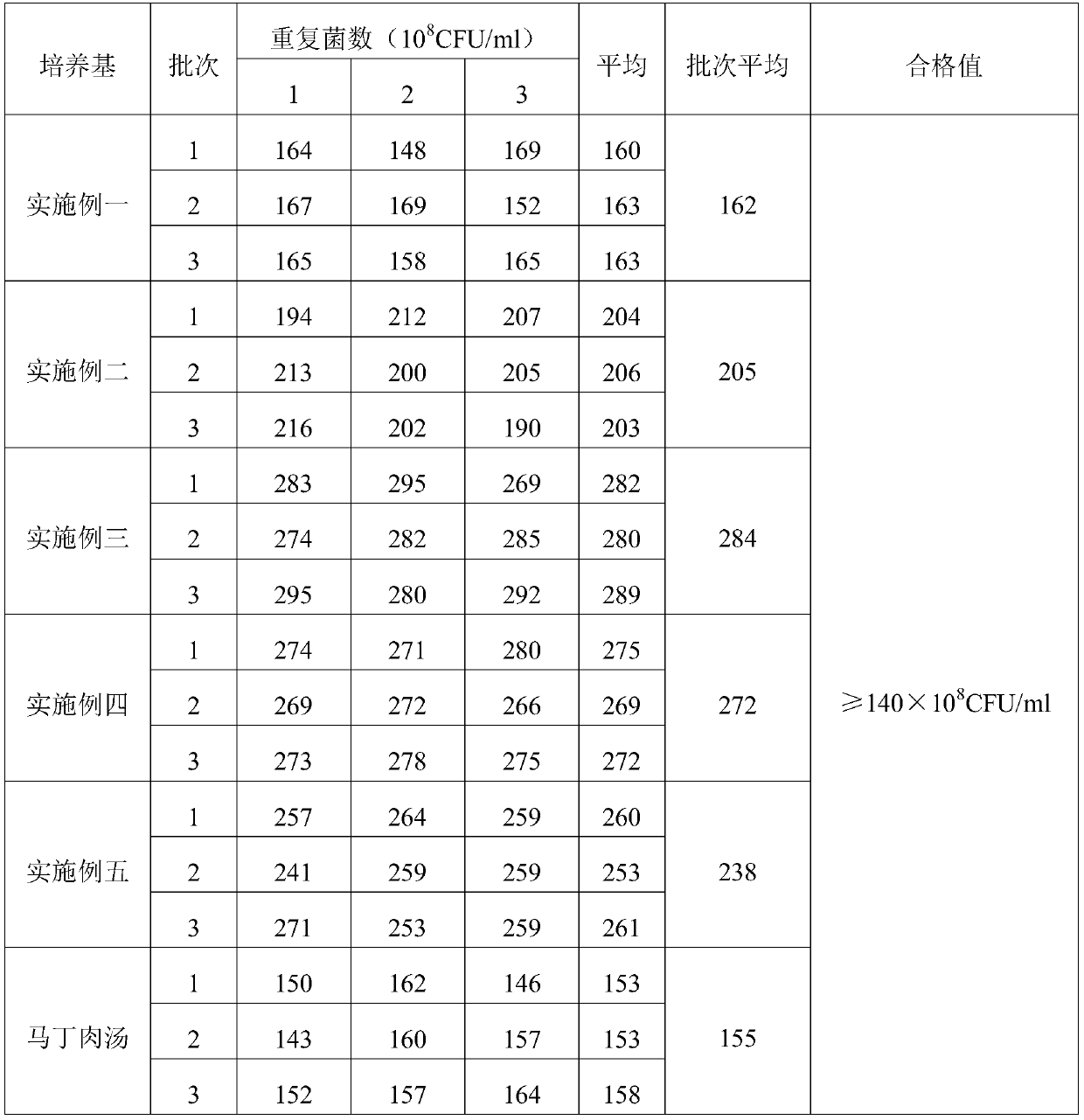
Embodiment 1
[0019] A porcine pneumonia vaccine enrichment medium, comprising the following raw materials in parts by weight, 10.0 g of meat liver and stomach membrane digestion liquid powder, 5.0 g of peptone, 3.0 g of NaCl, 7.0 g of bovine pancreas digest and 0.5 g of growth-promoting factor, purified water Add to 1000ml.
[0020] Wherein the growth-promoting factor comprises amino acid, trace salt ion and trace element, and described amino acid comprises L-glutamic acid 0.001g and p-aminobenzoic acid 0.001g, and described trace salt ion comprises dipotassium hydrogen phosphate 0.2674g, potassium dihydrogen phosphate 0.2000g, NH 4 Cl 0.01g, magnesium sulfate 0.01g and zinc sulfate 0.01g, said trace elements include niacin 0.0002g, calcium pantothenate 0.0002g, biotin 0.0002g.
[0021] Base preparation method: add all the raw materials used to prepare porcine pneumonia vaccine medium into purified water, fully dissolve, sterilize at 121°C for 15 minutes, and set aside.
Embodiment 2
[0023] A porcine pneumonia vaccine enrichment medium, comprising the following raw materials in parts by weight, 15.0 g of meat liver and gastric membrane digestion liquid powder, 8.0 g of peptone, 4.0 g of NaCl, 9.0 g of bovine pancreas digest and 0.8 g of growth-promoting factor, purified water Add to 1000ml.
[0024] Wherein the growth-promoting factor comprises amino acid, trace salt ion and trace element, and described amino acid comprises L-glutamic acid 0.003g and p-aminobenzoic acid 0.003g, and described trace salt ion comprises dipotassium hydrogen phosphate 0.3437g, potassium dihydrogen phosphate 0.3000g, NH 4 Cl 0.05g, magnesium sulfate 0.05g and zinc sulfate 0.05g, said trace elements include niacin 0.0001g, calcium pantothenate 0.0001g, biotin 0.0001g.
[0025] Base preparation method: add all the raw materials used to prepare porcine pneumonia vaccine medium into purified water, fully dissolve, sterilize at 121°C for 15 minutes, and set aside.
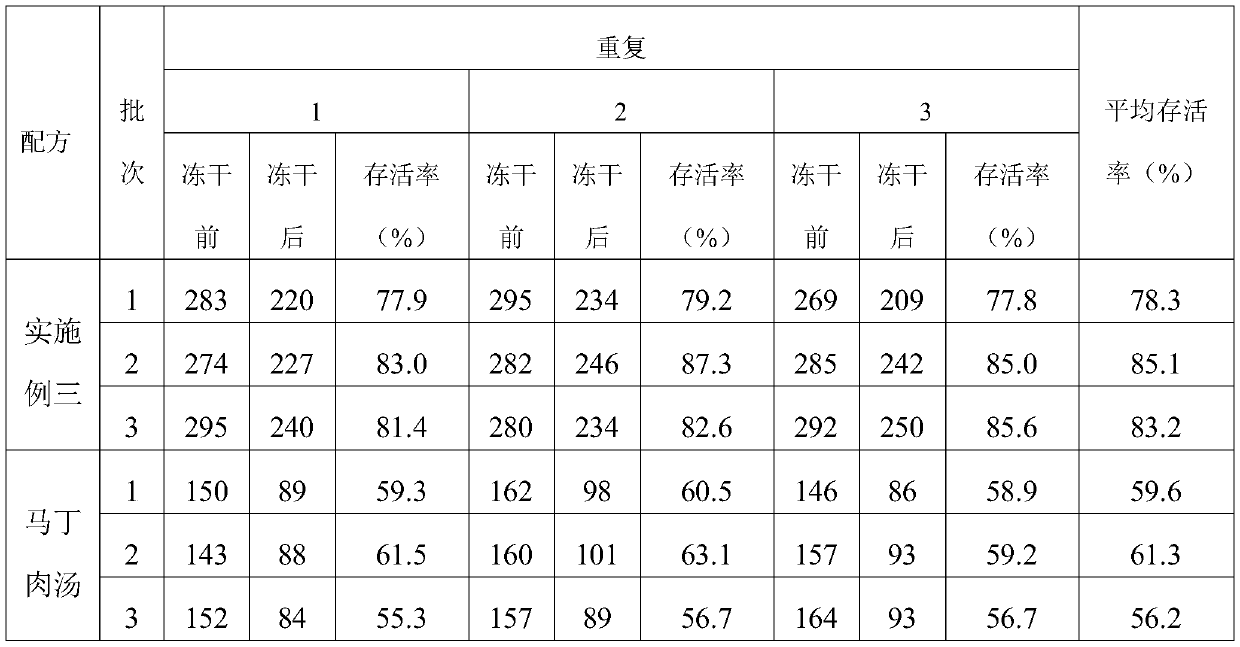
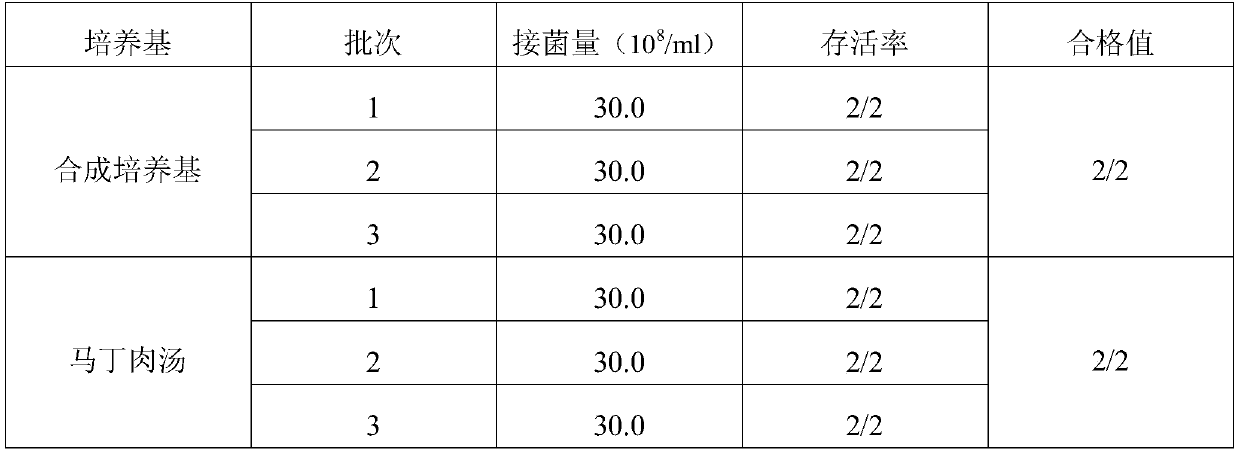
Embodiment 3
[0027] A porcine pneumonia vaccine enrichment medium, comprising the following raw materials in parts by weight, 20.0 g of meat liver and gastric membrane digestion liquid powder, 10.0 g of peptone, 5.0 g of NaCl, 8.0 g of bovine pancreas digest and 1.0 g of growth-promoting factor, purified water Add to 1000ml.
[0028] Wherein the growth-promoting factor comprises amino acid, trace salt ion and trace element, and described amino acid comprises L-glutamic acid 0.002g and p-aminobenzoic acid 0.002g, and described trace salt ion comprises dipotassium hydrogen phosphate 0.5g, potassium dihydrogen phosphate 0.4g, NH4Cl 0.03g, magnesium sulfate 0.033g and zinc sulfate 0.03g, the trace elements include niacin 0.001g, calcium pantothenate 0.001g, biotin 0.001g.
[0029] Base preparation method: add all the raw materials used to prepare porcine pneumonia vaccine medium into purified water, fully dissolve, sterilize at 121°C for 15 minutes, and set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com