Triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and preparation method of vaccine
A technology for rabbit viral hemorrhage and bordetella disease, applied in the direction of virus/phage, biochemical equipment and methods, virus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1 - construction of recombinant baculovirus VP60 strain
[0075] 1. Construction of transfer vector pVL-HBM
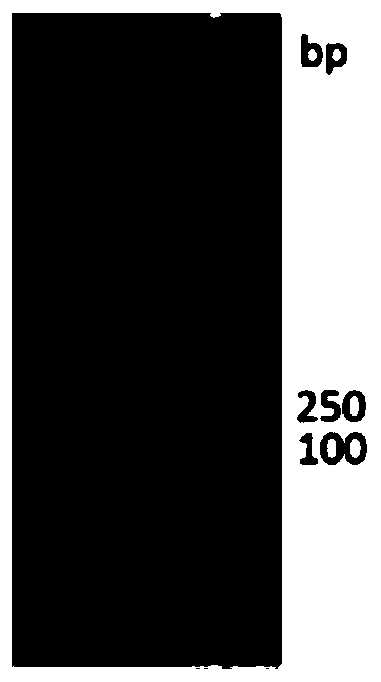
[0076] (1) HBM gene amplification HBM gene amplification uses pMD19-HBM as a template, and HBM-F and HBM-R as primers for PCR amplification ( figure 1 ).
[0077] HBM-F (sequence 1): 5'-agcggatcca aaccatgaaa ttc-3'(BamHI) 23
[0078] HBM-R (sequence 2): 5'-ataagatctg catgcggtac ccc-3'(PstⅠ)23
[0079] (2) Enzyme digestion The correctly identified PCR product was recovered by a DNA gel recovery kit, the pVL1393 plasmid and the purified HBM gene PCR product were digested with BamHI / PstI at 37°C for 3 hours, and the digested product was subjected to gel electrophoresis. Gel Recovery and Purification Kit for purification and recovery.
[0080](3) Ligation and transformation The digested products were subjected to gel electrophoresis, and purified and recovered using a gel recovery and purification kit. The digested and purified pVL1393 plasmid and th...
Embodiment 2
[0104] Embodiment 2——vaccine preparation
[0105] 1. Preparation of semi-finished product of recombinant baculovirus VP60 strain
[0106] (1) Propagation of virus seeds Inoculate the well-grown Sf9 cells with the basic virus seeds of the recombinant baculovirus VP60 strain, culture at 27°C for 72-96 hours, harvest the virus liquid, pass it on for 2 generations, harvest the virus liquid of the fourth generation, and use it as a production virus kind.
[0107] (2) Preparation of cells for production
[0108] 1) Cell recovery Take the frozen Sf9 cells out of the liquid nitrogen, put them into warm water at 37°C and shake quickly until the frozen solution is completely dissolved, transfer the Sf9 cell suspension to a centrifuge tube, centrifuge at 620r / min for 5min, discard For the supernatant, resuspend the cells with SF-SFM medium, place them in a Erlenmeyer flask with a suitable capacity, and culture them on a shaker at 27°C with a rotation speed of 100-105r / min.
[0109] 2)...
Embodiment 3
[0134] Embodiment 3 - the inspection of vaccine
[0135] 1. Properties After standing still, the upper layer is a clear liquid, and the lower layer has a small amount of precipitation. After shaking, it becomes a uniform suspension.
[0136] 2. Filling inspection and sterility inspection are carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and all of them meet the regulations.
[0137] 3. Safety inspection 3 batches of laboratory products were subcutaneously immunized healthy susceptible rabbits around 45 days old, 2.0ml / piece, and observed continuously for 10 days. The results showed that all the experimental rabbits were healthy and alive, and their spirit, diet, feces, and body temperature were all normal. ; There was no swelling or necrosis at the inoculation site, and no other abnormal clinical symptoms appeared. The specific results are shown in Table 1.
[0138] Table 1 Safety test results of 3 batches of laboratory products
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com