Mycoplasma bovis immune related protein, detection kit containing same and application thereof to detection of mycoplasma bovis antibody
A technology for detection of mycoplasma bovis and antibodies, applied in the biological field, can solve problems such as difficulties in disease prevention and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
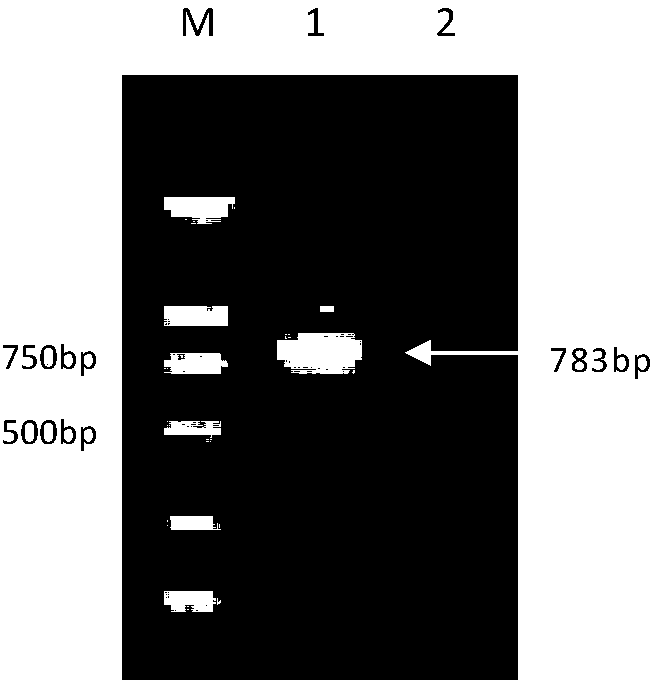
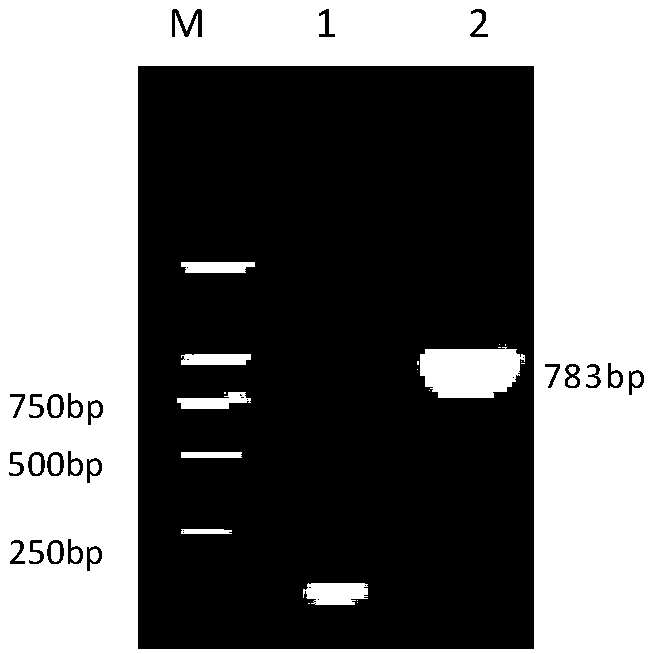
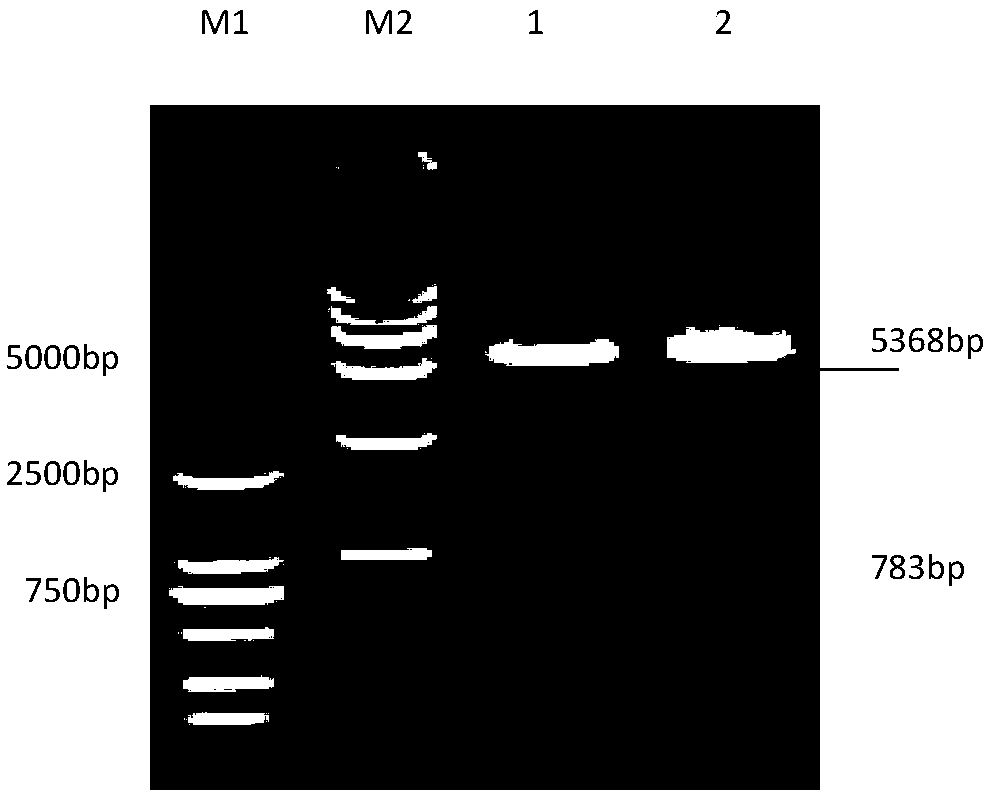
[0040] Example 1 Cloning and expression of the gene encoding the Mycoplasma bovis protein P28 protein
[0041] 1 material
[0042] 1.1 Strain, plasmid
[0043] M.bovis Hubei-1 isolate was isolated, identified and preserved in this experiment (Xin Jiuqing et al., 2008); pET-28aVector was purchased from Invitrogen. The whole genome sequence analysis of M.bovis Hubei-1 isolate was completed by our laboratory. Huashun DNA Column Gel Recovery Kit was purchased from Shanghai Huashun Biotechnology Co., Ltd. Competent cells DH5α and BL21(DE3) were preserved by our laboratory.
[0044] 1.2 Main reagents
[0045] TMB substrate chromogenic solution was purchased from Tianhe Biochemical Technology Co., Ltd.; restriction endonucleases BamH I, SalI, dNTP, and rTaq DNA polymerase were purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0046] 1.3 Main solution and preparation
[0047] Mycoplasma liquid medium: PPLO broth 21g, inactivated horse serum 200mL, fresh yeast extrac...
Embodiment 2
[0092] Example 2 Establishment of M.bovis antibody indirect ELISA method
[0093] Using the P28 recombinant protein prepared in Example 1 as an antigen, an ELISA method for M. bovis serum antibody was established. By optimizing the reaction conditions, determine the best conditions such as antigen coating concentration, primary antibody dilution, optimal blocking conditions, primary antibody action time, secondary antibody action time, color development time, and judgment of critical values. This test laid a theoretical foundation for the research of the indirect ELISA kit for the detection of Mycoplasma bovis antibody, and also provided a reference for the improvement of the field diagnosis method of M.bovis.
[0094] 1 material
[0095] 1.1 Main reagents
[0096] Fish gelatin was purchased from Sigma Company; Ammonium sulfate was purchased from Tianjin Chemical Reagent No. 1 Factory; horseradish peroxidase-labeled rabbit anti-bovine IgG antibody was purchased from Sigma Co...
Embodiment 3
[0172] Example 3 Performance evaluation of the MbH kit of the present invention and its comparison with other commercial kits
[0173] In order to speed up the industrialization of the MbH kit, the present invention evaluates the performance of the kit such as sensitivity, specificity, accuracy of detecting clinical samples, duration of host antibody and storage period of the kit. In addition, the MbH kit is compared with commercial kits to provide data reference for the application of the MbH kit.
[0174] 1 material
[0175] 1.1 ELISA kit
[0176] The "MbH M.bovis ELISA antibody detection kit" ("MbH kit" for short) of the present invention was prepared according to the method in Example 2, and the batches were: 201001, 201002, and 201003, with 20 kits in each batch.
[0177] "Mycoplasma bovis Antibody Test Kit (ELISA)" ("Kit 1" for short) was purchased from Biovet, Canada; "BIO-X MYCOPLASMA BOVIS ELISA KIT" ("Kit 2" for short) was purchased from Bio-X Diagnostics, Belgium....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com