Mycoplasma bovis multifunctional protein CDNPase
A technology of mycoplasma bovis and protein, applied in the direction of biochemical equipment and methods, applications, botanical equipment and methods, etc., can solve problems such as little known, undiscovered toxins, virulence factors and virulence islands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
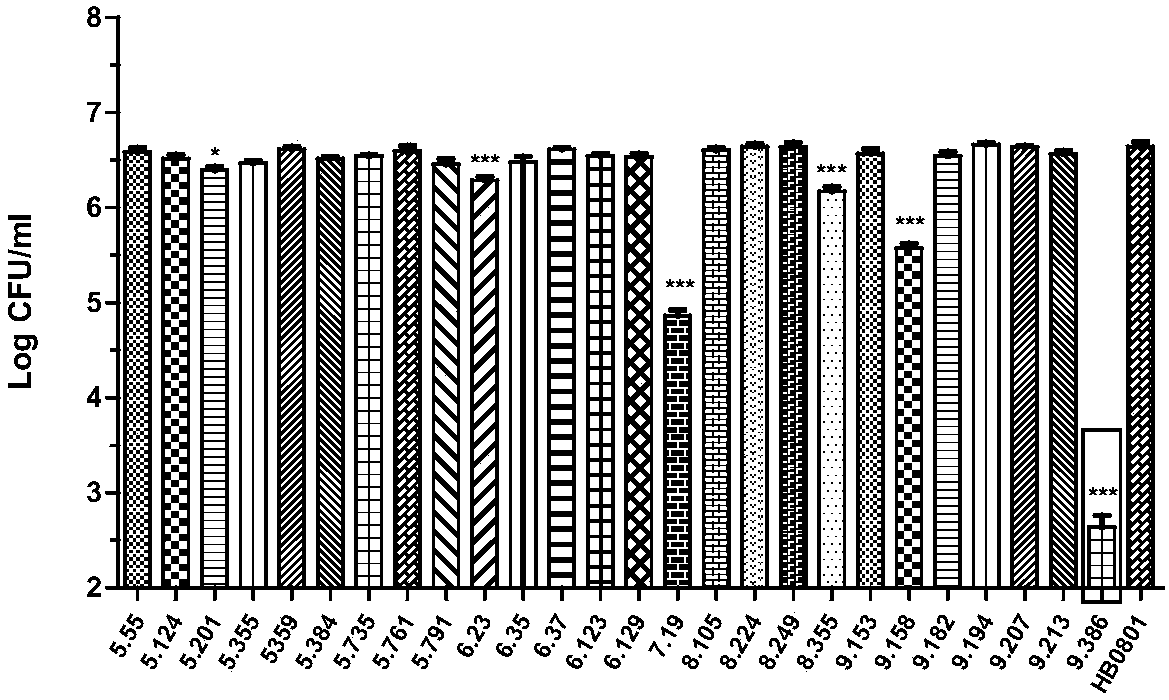
[0038] Example 1: Screening and identification of growth-deficient mutants of Mycoplasma bovis
[0039] (1) High-throughput screening of growth-deficient mutants of Mycoplasma bovis
[0040] Using the PEG8000-mediated transformation method, the Tn4001 transposon mutant library of Mycoplasma bovis HB0801 was successfully constructed (Mahairas and Minion, 1989). The mutant library contained a total of 2285 mutants, which had been stored separately for individual strains. High-throughput screening of the Mycoplasma bovis mutant library was performed using the growth-deficient experimental cell infection model and the 96-pin replicator constructed in our laboratory. The specific steps are as follows: spread bovine embryonic lung cells (Embryonic bovine lung, EBL) cells to 96-well cell culture plate according to 4 × 104 cells / cm2, and use 96-pin replicator to inoculate the mutant library into the wells of the cell culture plate respectively. Co-cultivate for 72 hours in a carbon d...
Embodiment 2
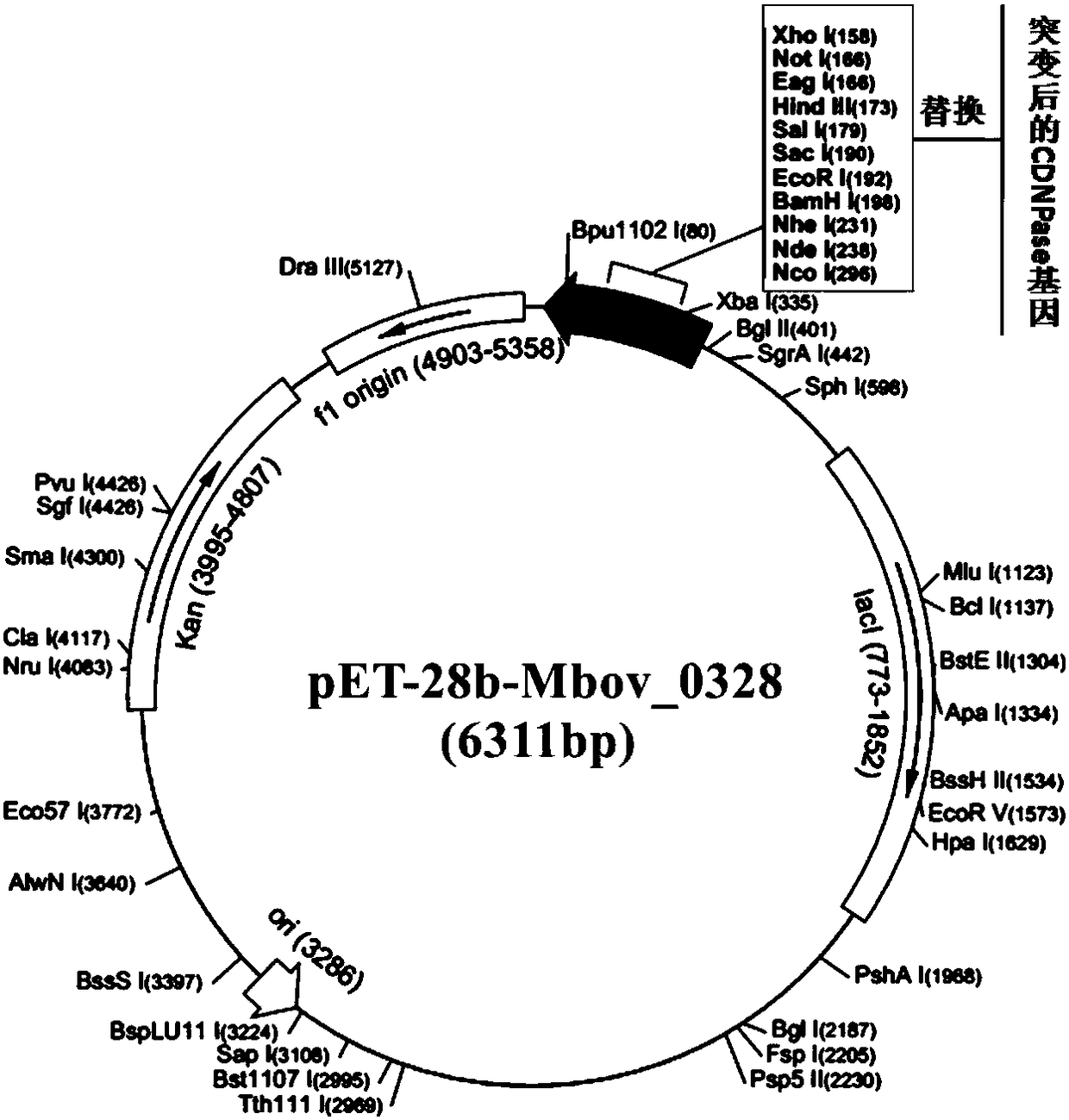
[0045] Embodiment 2: Expression of Mycoplasma bovis CDNPase protein
[0046] 1. Cloning and expression of Mycoplasma bovis Mbov_0328 gene
[0047] Because E. coli is to the preference of codon, the codon UGA of tryptophan of encoding tryptophan in E. coli is used as terminator in E. coli in the present invention, therefore, when expressing M. bovis gene with E. coli, need carry out mycoplasma gene Mutation, the codon UGA is mutated to the codon UGG that can express tryptophan in E. coli.
[0048] The applicant obtained a local isolate of Mycoplasma bovis from the lung tissue of a cattle farm in Yingcheng City, Hubei Province in June 2008, named it Mycoplasma bovis HB0801, Mycoplasma bovis HB0801, and sent it on February 1, 2010 It was deposited in the Chinese Type Culture Collection Center of Wuhan University, China. The deposit number is CCTCC NO: M2010040. The present invention utilizes self-designed PCR primers to mutate the gene, and the specific steps are: take the Mbov...
Embodiment 3
[0084] Example 3: Multifunctional Verification of Mycoplasma bovis rCDNPase Recombinant Protein
[0085] 1. Phosphodiesterase Activity Test of Mycoplasma bovis rCDNPase Recombinant Protein
[0086] (1) Reaction system preparation: Add the following reaction solution to a 1.5ml EP tube, Tris-HCL (pH 7.0) 100mM, Mncl 2 10mM, substrate cyclic dinucleotide (cyclic diadenylic acid c-di-AMP and cyclic diguanylic acid c-di-GMP) 50μM, rCDNPase recombinant protein 5μM, the total reaction system is 100mL. Mix the prepared reaction system Evenly, react at 37°C.
[0087] (2) Determination of phosphodiesterase activity: the sample was detected by HPLC separation and detection method, and the specific settings were as follows: 10% methanol and 0.2% ammonium acetate were used as the mobile phase, the flow rate was 1mL / min, and the column oven was controlled at 25°C , the injection volume of the autosampler was 10 μL, the reaction products were separated, and the wavelength of the ultraviol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com