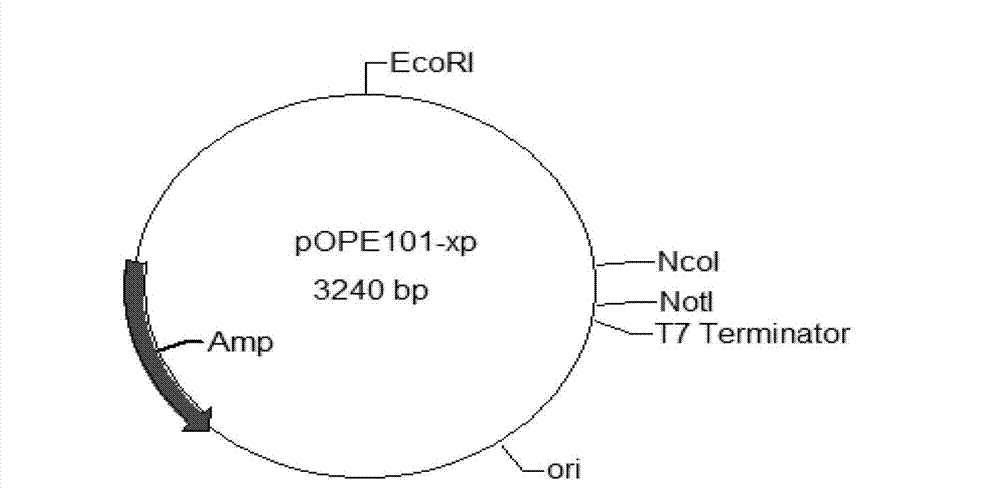
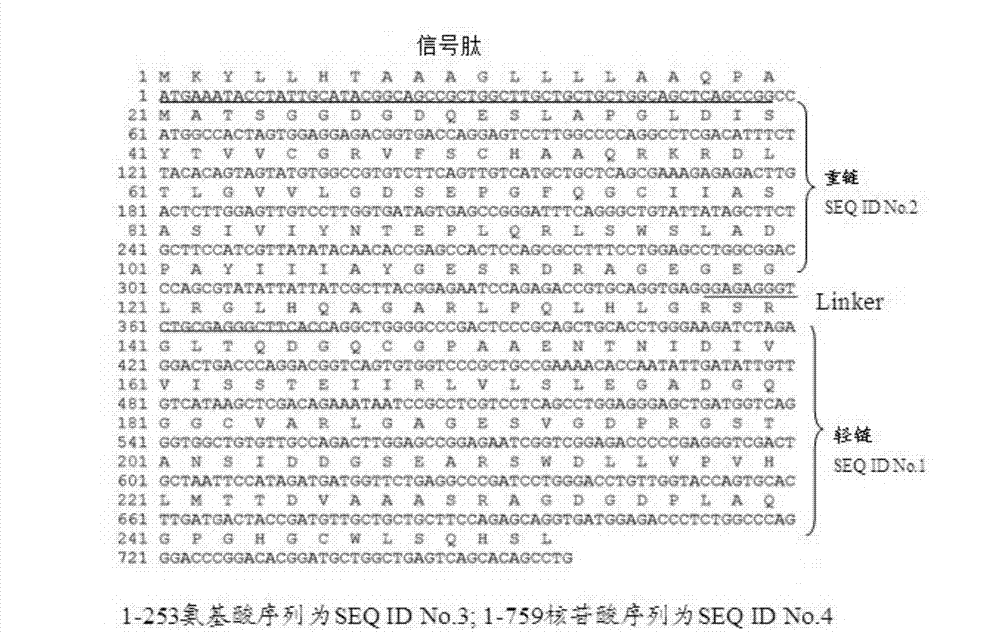
Bovine-derived anti-staphylococcus aureus single-chain antibody, preparation method and application thereof
A single-chain antibody and Staphylococcus technology, applied in the field of genetic engineering, can solve problems such as difficult to cure and easy to produce resistance to antibiotics, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of Bovine Anti-Mastitis Single Chain Antibody
[0029] 1: Collect the blood of dairy cows suffering from mastitis, and when the serum antibody titer detected by ELISA is greater than 1:20000, continue the follow-up experiment. The anticoagulated blood was extracted from the peripheral nuclear monocytic cells, and the total RNA was extracted by the Trizol method (TRIZOL Reagent was purchased from TaKaRa Company). Using the extracted total RNA as a template, Oligo primer was used to synthesize the first-strand cDNA according to the product instructions of the reverse transcription kit (cDNA first-strand synthesis kit was purchased from TaKaRa Company).
[0030] 2: Design primers for amplifying the light and heavy chains of the antibody (Table 1) according to the FR region of the variable region sequence of the bovine antibody coding gene (Madhuri Kotia, 2010 Molecular Immunology; 2011, vaccine) published in the literature (Table 1), where VH1F and VH1R are use...
Embodiment 2
[0042] Indirect ELISA Screening of Antigen-specific ScFv
[0043] Prepare the whole bacterial antigen of Staphylococcus aureus (ATCC-25923) and coat it with 50mmol / L sodium bicarbonate solution (pH9.6) at 4°C overnight, block with 5% skimmed milk powder solution for 1h, and then use PBST (containing 0.1% Tween20, The same below) and wash 3 times; take 50 μL of the supernatant of the TES-treated bacteria liquid, mix it with 50 μL of 4% skimmed milk powder solution, add the above-mentioned coated Staphylococcus aureus antigen, react at 37 ° C for 2 hours, and wash with PBST; add Myc -Tag Mouse mAb (Myc-tag mouse monoclonal antibody purchased from RayBioTech Company) 100 μL (1:2000) reacted at 37°C for 2 h, washed with PBST; added Peroxidase-conjugated Affinipure GoagtAnti-Mouse IgG (purchased from Abmart Company) 100 μL (1:2000) 4000), react at 37°C for 1 h, wash with PBST; develop color with TMB, stop the reaction with 2mol / L sulfuric acid, and read the OD450 value with a micro...
Embodiment 3
[0046]
[0047]
[0048]
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com