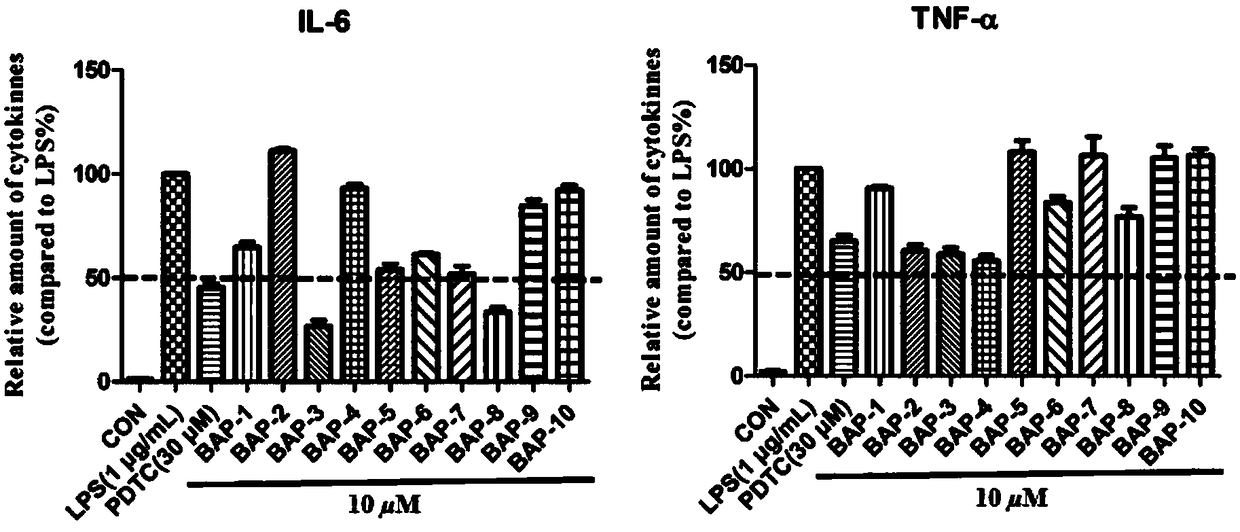
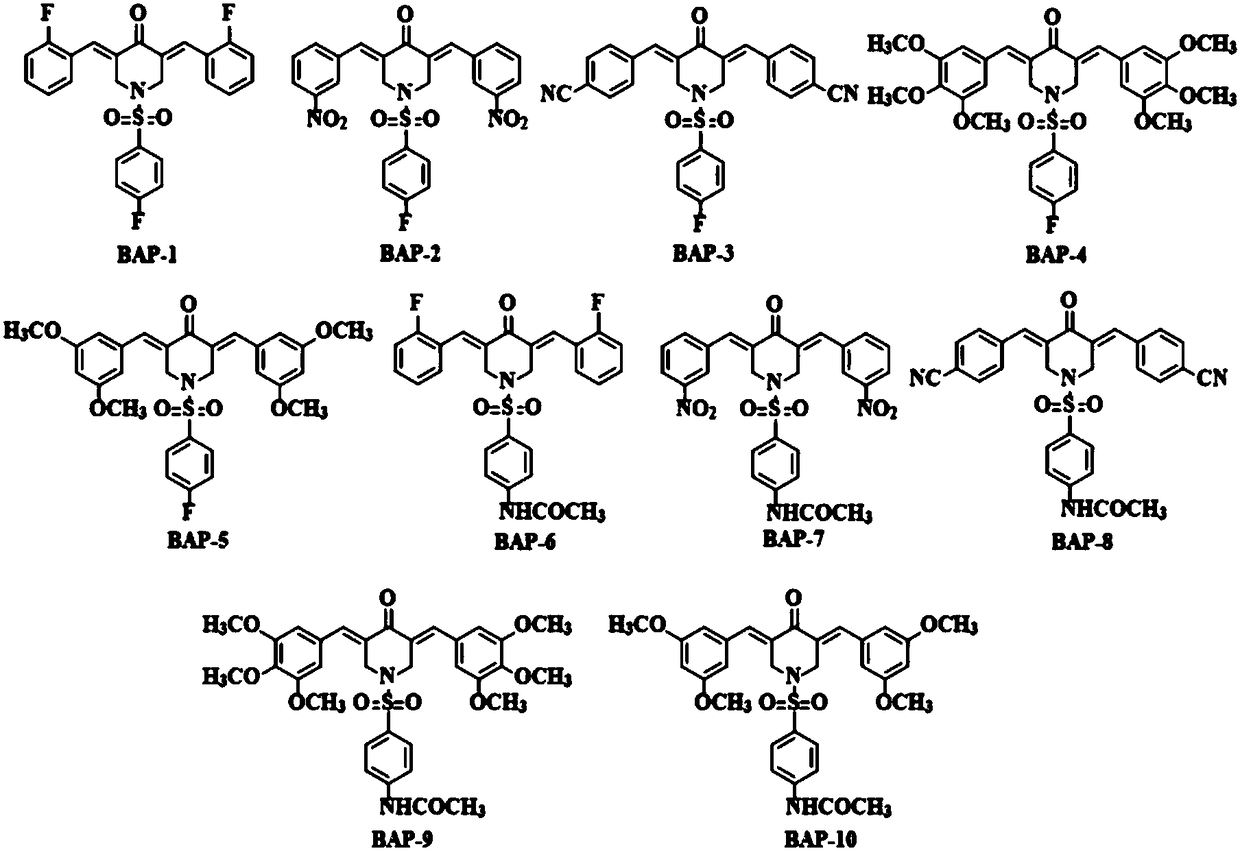
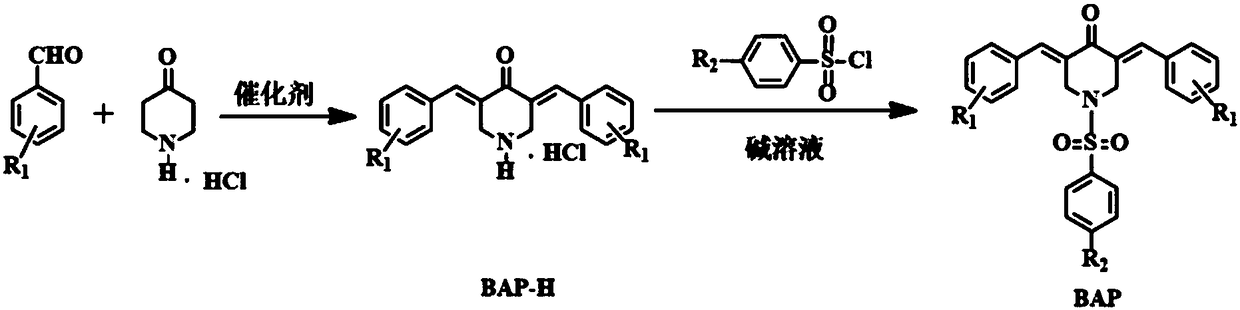
3,5-bis(arylidene)-N-benzenesulfonyl-4-piperidinone compounds and preparation method thereof
A technology of diaryl methylene and acetamidobenzenesulfonyl, which is applied in the field of anti-tumor and anti-inflammatory drugs and their preparation, can solve the problems of less research on benzenesulfonylation modification, and achieve favorable promotion and mild reaction conditions , the effect of high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 3,5-bis(2-fluorobenzylidene)-N-(4-fluorobenzenesulfonyl)-4-piperidone (BAP-1)
[0033] Mix 0.01mol of 4-piperidone hydrochloride and 0.02mol of 2-fluorobenzaldehyde in a solution of 15mL of methanol and water, add 2-3mL of 20% sodium hydroxide solution dropwise at room temperature, and stir at 50°C for 6 hours , The end point of the reaction was determined by thin layer chromatography (TLC). After the reaction, the precipitate was suction filtered, and the resulting precipitate was washed with 70% aqueous methanol to obtain a yellow solid, namely the intermediate BAP-H (1). Then, the intermediate BAP-H (1) and 4-fluorobenzenesulfonyl chloride were dissolved in 10 mL Add 3-5 drops of pyridine to dichloromethane, stir overnight at room temperature, and determine the reaction end point by thin layer chromatography (TLC). The reaction solution was washed twice with 2mol / L=10:10:1) hydrochloric acid solution, and the reaction solution was concentrated under red...
Embodiment 2
[0036] Synthesis of 3,5-bis(3-nitrobenzylidene)-N-(4-fluorobenzenesulfonyl)-4-piperidone (BAP-2)
[0037] Mix 0.01mol of 4-piperidone hydrochloride and 0.02mol of 3-nitrobenzaldehyde in a solution of 15mL of methanol and water, add 2-3mL of 20% sodium hydroxide solution dropwise at room temperature, and stir at 40°C The reaction was carried out for 6 hours, and the end point of the reaction was determined by thin layer chromatography (TLC). After the reaction was completed, the precipitate was suction-filtered, and the resulting precipitate was washed with 70% aqueous methanol to obtain a yellow solid, the intermediate BAP-H (2). Then, the intermediate BAP-H (2) and 4-fluorobenzenesulfonyl chloride were dissolved in 10 mL Add 3-5 drops of pyridine to dichloromethane, stir overnight at room temperature, and determine the reaction end point by thin layer chromatography (TLC). The reaction solution was washed twice with 2mol / L hydrochloric acid solution, concentrated under reduc...
Embodiment 3
[0040] Synthesis of 3,5-bis(4-cyanobenzylidene)-N-(4-fluorobenzenesulfonyl)-4-piperidinone (BAP-3)
[0041] 0.01mol of 4-piperidone hydrochloride and 0.02mol of 4-cyanobenzaldehyde were mixed in 10mL of acetic acid, and dry hydrogen chloride gas was continuously introduced for 45min, stirred and reacted at 25°C for 8 hours, and passed thin layer chromatography ( TLC) to determine the end point of the reaction. After the reaction, the precipitate was suction filtered, the precipitate was dissolved in water and the pH value was adjusted to neutral with sodium hydroxide solution, and the resulting precipitate was washed with 70% methanol aqueous solution to obtain a yellow solid, which was the intermediate BAP-H (3). Then, the intermediate BAP-H(3) and 4-fluorobenzenesulfonyl chloride were dissolved in 10 mL of dichloromethane, 3-5 drops of pyridine were added, stirred at room temperature overnight, and the end point of the reaction was determined by thin layer chromatography (TL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com